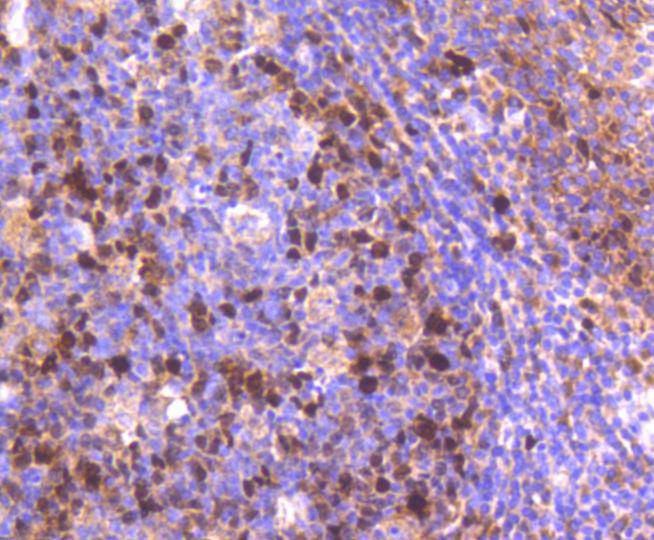
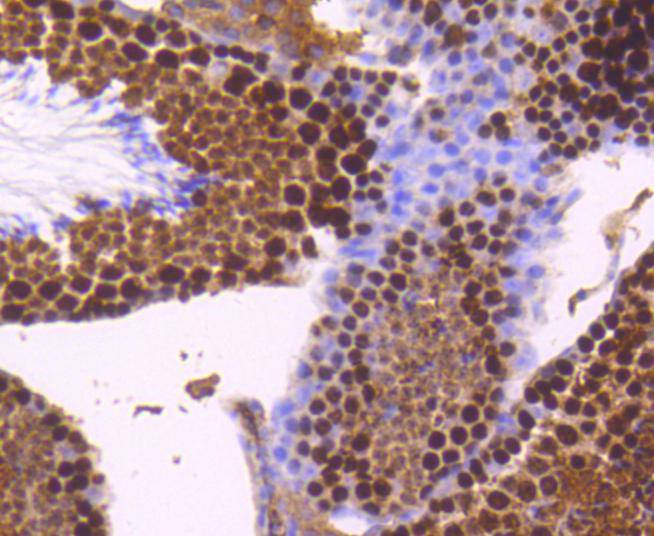
DNA topoisomerase I and II (Topo I and Topo II) are nuclear enzymes that regulate the topological structure of DNA in eukaryotic cells by transiently breaking and rejoining DNA strands. Eukaryotic topoisomerases are capable of relaxing both positive and negative supercoils, whereas prokaryotic topoisomerases relax only negative supercoils. DNA topoisomerases play a role in DNA replication, recombination, and transcription and have been identified as targets of numerous anticancer drugs. Topo I, a ubiquitously expressed, soluble enzyme, acts by introducing a transient break in one strand of DNA, while Topo II acts by making a transient double-strand break. Topo II is encoded by two different genes to generate two distinct isoforms that are designated Topo IIα and Topo IIβ. Topo IIβ and Topo IIα, are largely homologous at their N-terminal three quarters, however, the C-terminal segments are considerably divergent, suggesting that these regions may mediate different cellular functions and account for the observed differential tissue expression patterns of the two isoforms.