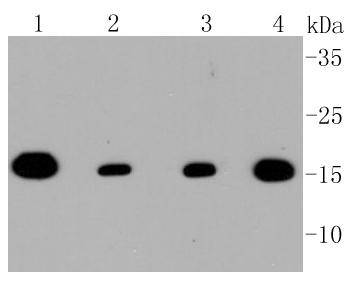
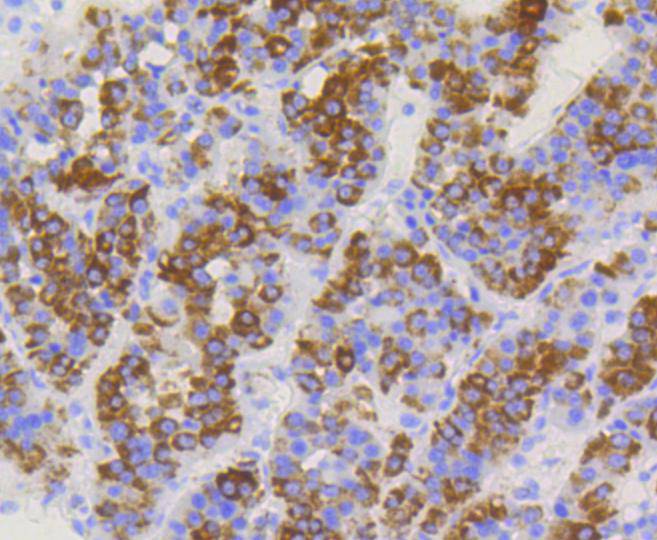
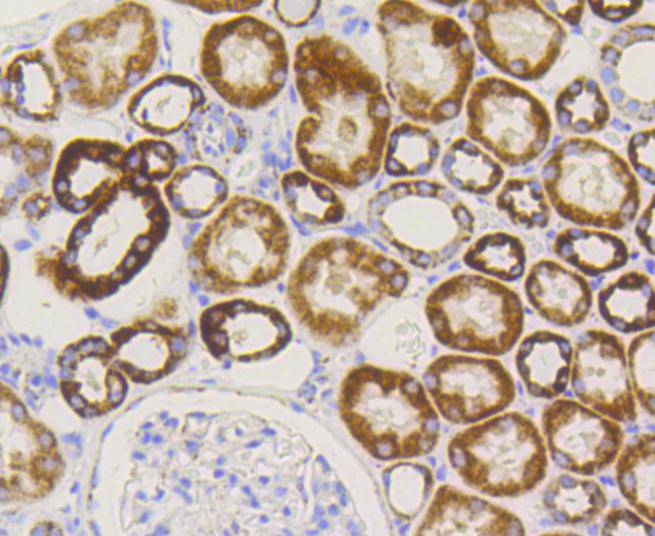
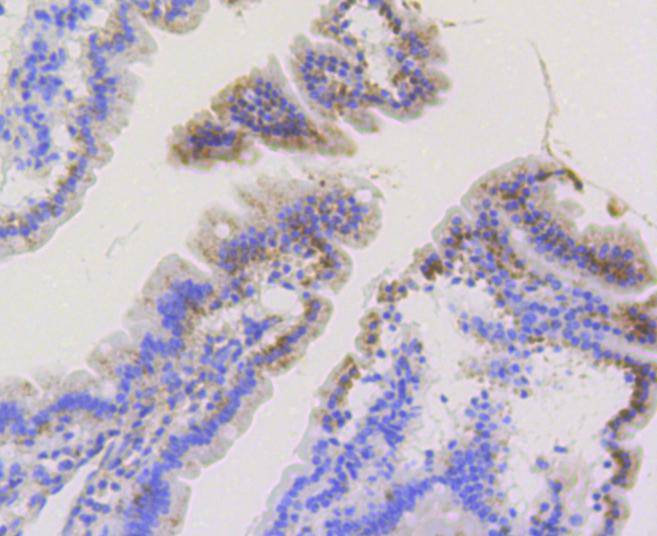
The mitochondrial preprotein translocases of the outer membrane (Tom) is a multisubunit protein complex that facilitates the import of nucleus-encoded precursor proteins across the mitochondrial outer membrane. The Tom machinery consists of import receptors for the initial binding of cytosolically synthesized preproteins and a general import pore (GIP) for the membrane translocation of various preproteins into the mitochondria. The import receptors include Tom20 and Tom22, which form a heteromeric receptor complex that initiates the insertion of newly synthesized proteins into the outer membrane and then directs the precursor protein into the GIP. In yeast, Tom22 is the essential component of the import receptor complex as it functions as both a receptor for the preproteins and serves as a docking point for both Tom20 and the GIP. Tom22 directly associates with Tom40, the major component of the GIP, and thereby forms a stable interaction between the two core complexes to facilitate the fluid movement of preproteins into the mitochondria. The insertion of Tom40 into the Tom machinery requires the initial binding of Tom40 to Tom20 and leads to the efficient incorporation of Tom40 precursors into preexisting Tom complexes.