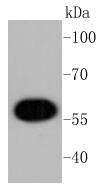
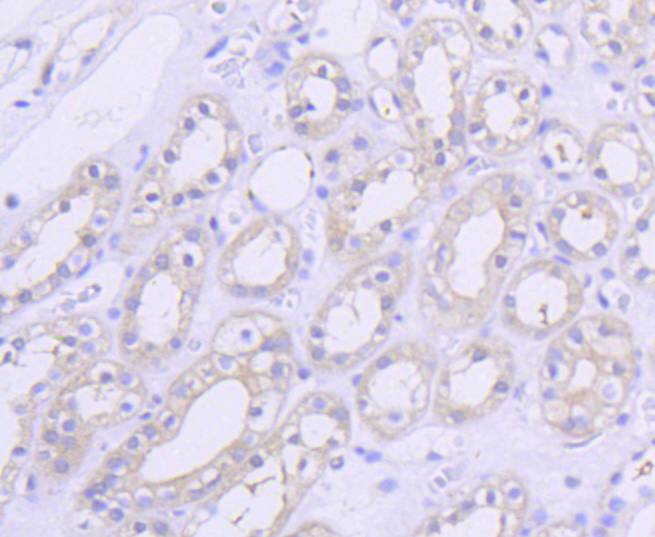
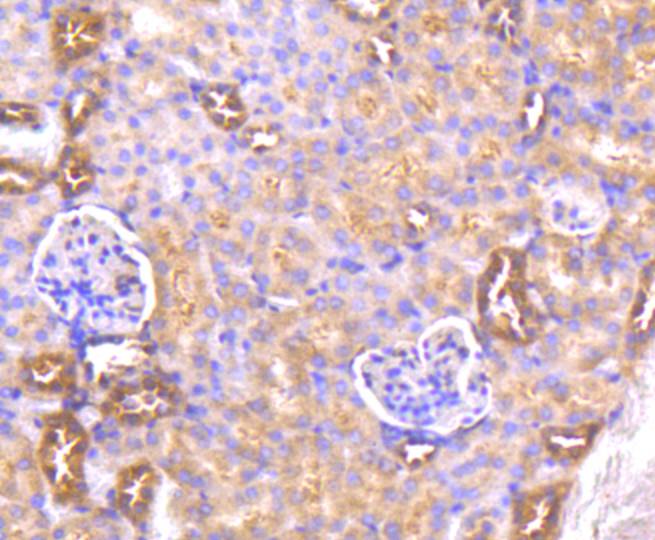
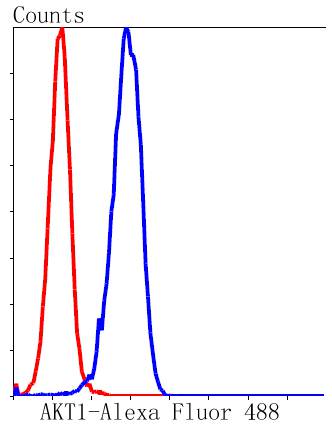
The serine/threonine kinase Akt family contains several members, including Akt1 (also designated PKB or RacPK), Akt2 (also designated PKBβor RacPK-β) and Akt 3 (also designated PKB? or thyoma viral proto-oncogene 3), which exhibit sequence homology with the protein kinase A and C families and are encoded by the c-Akt proto-oncogene. All members of the Akt family have a pleckstrin homology domain. Akt1 and Akt2 are activated by PDGF stimulation. This activation is dependent on PDGFR-β tyrosine residues 740 and 751, which bind the subunit of the phosphatidylinositol 3-kinase (PI 3-kinase) complex. Activation of Akt1 by insulin or insulin-growth factor-1(IGF-1) results in phosphorylation of both Thr 308 and Ser 473. Phosphorylation of both residues is important to generate a high level of Akt1 activity, and the phosphorylation of Thr 308 is not dependent on phosphorylation of Ser 473 in vivo. Thus, Akt proteins become phosphorylated and activated in insulin/IGF-1-stimulated cells by an upstream kinase(s). The activation of Akt1 and Akt2 is inhibited by the PI kinase inhibitor wortmannin, suggesting that the protein signals downstream of the PI kinases.