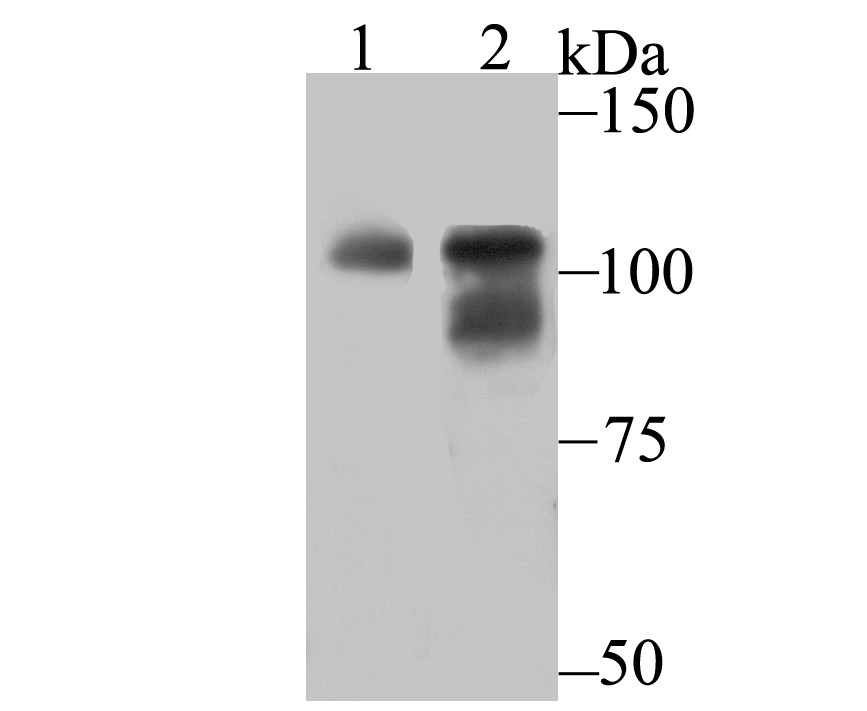
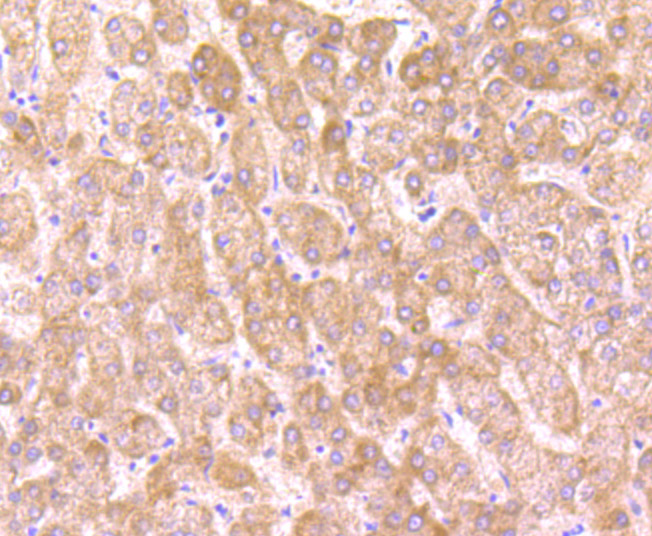
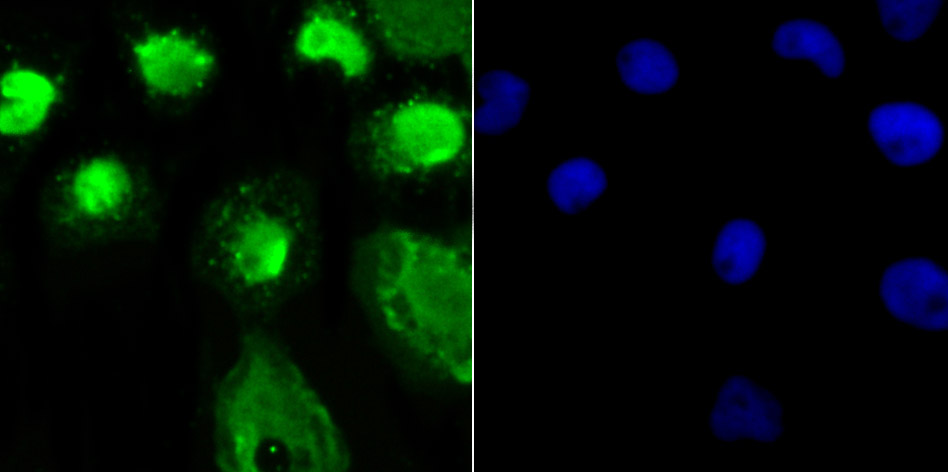
O-linked N-acetylglucosamine (O-GlcNAc) transferase (also designated OGT) catalyzes the addition of a single N-acetylglucosamine in O-glycosidic linkage to serine or threonine residues. Since both phosphorylation and glycosylation compete for similar serine or threonine residues, the two processes may compete for sites, or they may alter the substrate specificity of nearby sites by steric or electrostatic effects. O-GlcNAc transferase has been purified from rat liver. It exists as a heterotrimeric complex with two subunits of the same molecular mass and one shorter subunit. Both polypeptides are related; the short subunit band is either a proteolytic product of the polypeptide or the product of an alternative translation start site. O-GlcNAc transferase is expressed as multiple transcripts that are present in different amounts in various human tissues, with the highest levels of expression in pancreas. Immunofluorescence of human cells expressing rat O-GlcNAc transferase indicated that it is present in both the nucleus and cytosol. HeLa cells expressing O-GlcNAc transferase do not survive well during prolonged incubations, suggesting that this protein may be toxic to the cells.