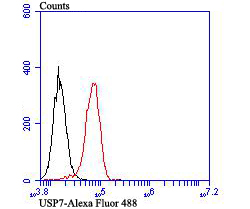
HAUSP (herpesvirus-associated ubiquitin-specific protease, USP7) is a ubiquitin-specific protease. HAUSP localizes predominantly to the nucleus, in a TD-dependent manner, where it associates with ND10. ND10 are small nuclear structures implicated in a variety of cellular processes including response to stress and interferons, oncogenesis, and viral infection. HAUSP binds strongly to Vmw110, a herpesvirus regulatory protein which has the ability to disrupt ND10. HAUSP, a novel p53-interacting protein, functions to deubiquitinize p53 in an important pathway for p53 stabilization. HAUSP strongly stabilizes p53 even in the presence of excess Mdm2, and also induces p53-dependent cell growth repression and apoptosis. The HAUSP protein is distributed in the nucleus in a micropunctate pattern with a limited number of larger discrete foci, some of which co-localize with PML in ND10. The gene encoding HAUSP maps to human chromosome band 16p13.2.