Product Detail
Product NameGRP78 BiP Antibody
Clone No.27
Host SpeciesMouse
ClonalityMonoclonal
PurificationProA affinity purified
ApplicationsWB, ICC, IHC
Species ReactivityHu
Immunogen Descpeptide
ConjugateUnconjugated
Other Names78 kDa glucose regulated protein antibody
78 kDa glucose-regulated protein antibody
AL022860 antibody
AU019543 antibody
BIP antibody
D2Wsu141e antibody
D2Wsu17e antibody
Endoplasmic reticulum lumenal Ca(2+)-binding protein grp78 antibody
Endoplasmic reticulum lumenal Ca2+ binding protein grp78 antibody
Epididymis secretory sperm binding protein Li 89n antibody
FLJ26106 antibody
Glucose Regulated Protein 78kDa antibody
GRP 78 antibody
GRP-78 antibody
GRP78 antibody
GRP78_HUMAN antibody
Heat shock 70 kDa protein 5 antibody
Heat Shock 70kDa Protein 5 antibody
Heat shock protein family A (Hsp70) member 5 antibody
HEL S 89n antibody
Hsce70 antibody
HSPA 5 antibody
HSPA5 antibody
Immunoglobulin Heavy Chain Binding Protein antibody
Immunoglobulin heavy chain-binding protein antibody
mBiP antibody
MIF2 antibody
Sez7 antibody
Accession NoSwiss-Prot#:P11021
Uniprot
P11021
Gene ID
3309;
Calculated MW78kDa
Formulation1*TBS (pH7.4), 0.5%BSA, 40%Glycerol. Preservative: 0.05% Sodium Azide.
StorageStore at -20˚C
Application Details
WB: 1:5,000-1:10,000
ICC: 1:200
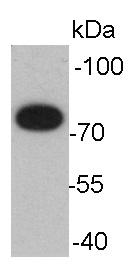
Western blot analysis on human spermatozoa lysates using anti- GRP78 mouse mAb.
ICC staining GRP78 in Hela cells (red). Cells were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS.
Binding immunoglobulin protein (BiP) also known as 78 kDa glucose-regulated protein (GRP-78) or heat shock 70 kDa protein 5 (HSPA5) is a protein that in humans is encoded by the HSPA5 gene. BiP is a HSP70 molecular chaperone located in the lumen of the endoplasmic reticulum (ER) that binds newly synthesized proteins as they are translocated into the ER, and maintains them in a state competent for subsequent folding and oligomerization. BiP is also an essential component of the translocation machinery, as well as playing a role in retrograde transport across the ER membrane of aberrant proteins destined for degradation by the proteasome. Like many stress and heat shock proteins, BiP/GRP78 has potent immunological activity when released from the internal environment of the cell into the extracelluar space.specifically, it feeds anti-inflammatory and pro-resolutory signals into immune networks, thus helping to resolve inflammation.
If you have published an article using product 48115, please notify us so that we can cite your literature.



 Yes
Yes



