Product Detail
Product NameAngiopoietin-2 Antibody
Clone No.4A2
Host SpeciesMouse
ClonalityMonoclonal
PurificationProA affinity purified
ApplicationsWB, IP, IF, IHC
Species ReactivityHu, Ms, Rt
Immunogen Descpeptide
ConjugateUnconjugated
Other NamesAGPT 2 antibody Agpt2 antibody ANG 2 antibody ANG-2 antibody ANG2 antibody Angiopoietin 2a antibody Angiopoietin 2B antibody Angiopoietin-2 antibody Angiopoietin2 antibody ANGP2_HUMAN antibody ANGPT 2 antibody Angpt2 antibody Tie2 ligand antibody
Accession NoSwiss-Prot#:O15123
Uniprot
O15123
Gene ID
285;
Calculated MW62-70kDa
Formulation1*TBS (pH7.4), 1%BSA, 40%Glycerol. Preservative: 0.05% Sodium Azide.
StorageStore at -20˚C
Application Details
WB: 1:100-1:1,000
IHC: 1:50-500
IP: 1-2 μg per 100-500 μg of total protein(1 ml of cell lysate)
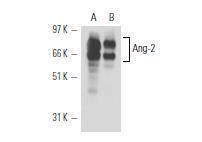
Western Blot analysis of Ang-2 expression in HUV-EC-C (A) and TF-1 (B) whole cell lysates.
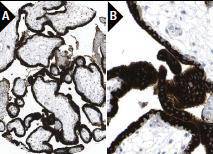
Immunoperoxidase staining of formalin fixed, paraffin-embedded human placenta tissue showing cytoplasmic staining of trophoblastic cells at low (A) and high (B) magnification.
Tie-1 and Tie-2 (also designated Tek) are novel cell surface receptor tyrosine kinases. The extracellular domain of Tie-1 has an unusual multidomain structure consisting of a cluster of three epidermal growth factor homology motifs localized between two immunoglobulin-like loops, which are followed by three Fibronectin type III repeats next to the transmembrane region. Angiopoietin-1 (Ang-1) is a secreted ligand for Tie-2. Preliminary biochemical analyses of Ang-1 reveal a potential Fibrinogen-like domain at the carboxy-terminus and coiled-coil regions in the amino-terminus. Ang-1 is an angiogenic factor that is thought to be involved in endothelial development. A related protein, angiopoietin-2 (Ang-2), has been identified as a naturally occurring antagonist of Ang-1 activation of Tie-2. In adult tissue, Ang-2 expression seems to be restricted to sites of vascular remodeling.
If you have published an article using product 48274, please notify us so that we can cite your literature.


 Yes
Yes



