Product Detail
Product NameRb2 p130 (Phospho-Thr986) Rabbit mAb
Clone No.S06-9B3
Host SpeciesRecombinant Rabbit
ClonalityMonoclonal
IsotypeRabbit IgG
PurificationAffinity Purified
ApplicationsWB
Species ReactivityHuman,Mouse
Immunogen DescA synthetic phosphopeptide corresponding to residues surrounding Thr986 of human p130
ConjugateUnconjugated
Other NamesRb2; P130
Accession NoSwiss-Prot:Q08999
GeneID:5934
Uniprot
Q08999
Gene ID
5934
Calculated MWPredicted band size: 128 kDa
Sdspage MWObserved band size: 130kDa
Concentration0.3 mg/ml
Formulation50mM Tris-Glycine(pH 7.4), 0.15M NaCl, 40% Glycerol, 0.01% Sodium azide and 0.05% BSA
StorageStore at 4˚C short term. Aliquot and store at -20˚C long term. Avoid freeze/thaw cycles.
Application Details
WB: 1:500-1:2000
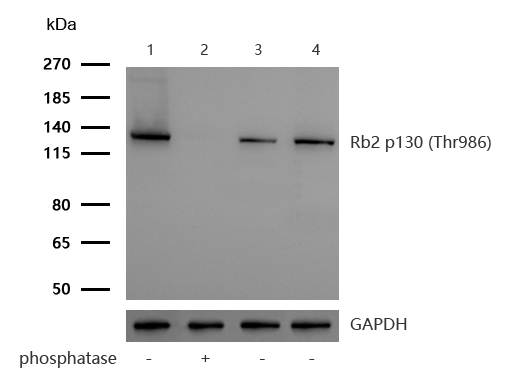
All lanes : Rb2 p130 (Phospho-Thr986) Rabbit mAb at 1/1k dilutionLane 1 : K562 whole cell lysatesLane 2 : K562 treated with Lambda Protein Phosphatase for 30min whole cell lysates/br>Lane 3 : JK whole cell lysatesLane 4 : C2C12 whole cell lysatesLysates/proteins at 20 µg per lane.SecondaryAll lanes : Goat Anti-Rabbit IgG H&L (HRP) at 1/20000 dilutionPredicted band size: 128 kDa Observed band size: 130kDaExposure time: 3 seconds
Swiss-Prot Acc.Q08999.Key regulator of entry into cell division. Directly involved in heterochromatin formation by maintaining overall chromatin structure and, in particular, that of constitutive heterochromatin by stabilizing histone methylation. Recruits and targets histone methyltransferases KMT5B and KMT5C, leading to epigenetic transcriptional repression. Controls histone H4 'Lys-20' trimethylation. Probably acts as a transcription repressor by recruiting chromatin-modifying enzymes to promoters. Potent inhibitor of E2F-mediated trans-activation, associates preferentially with E2F5. Binds to cyclins A and E. Binds to and may be involved in the transforming capacity of the adenovirus E1A protein. May act as a tumor suppressor.
If you have published an article using product 52095, please notify us so that we can cite your literature.


 Yes
Yes



