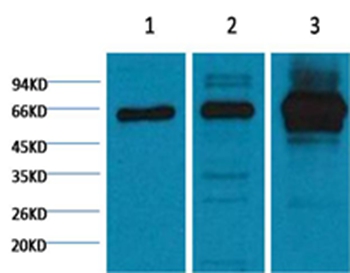
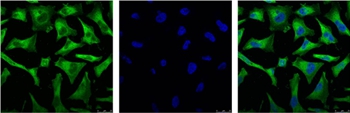
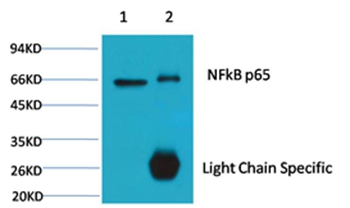
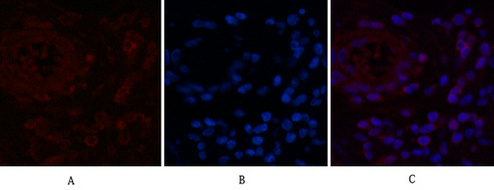
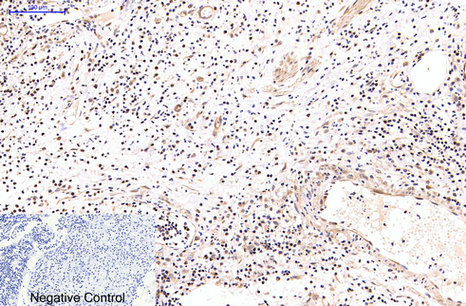
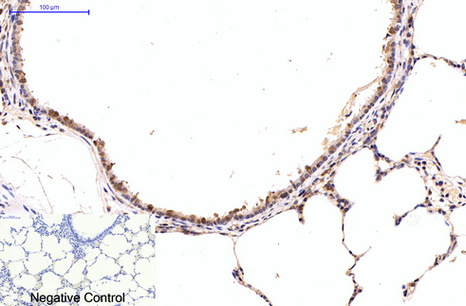
NFkB p65 is ubiquitinated leading to its proteosomal degradation, which is required for termination of the NFkB response. Phosphorylation of NFkB p65 on S536 stimulates acetylation of K310 by CBP, enhancing transcriptional activity. NFkB p65 is also acetylated at K122, enhancing DNA binding and impairing the interaction with NFKBIA. The protein is deacetylated by HDAC3. Invasion of a host by a pathogen is frequently associated with the activation of NF-kB, which coordinates various aspects of immune function required for resistance to infection.