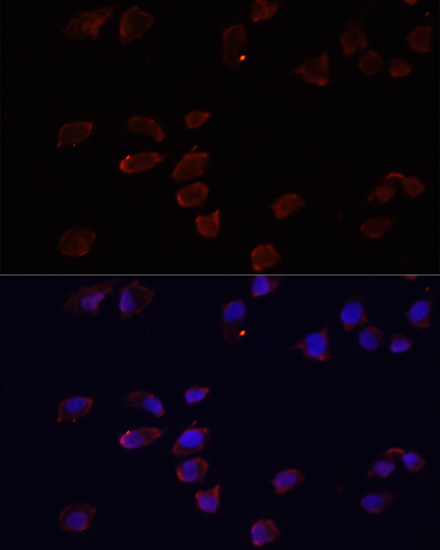
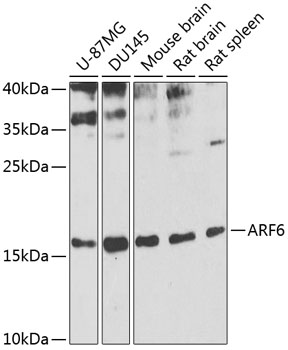
ADP-ribosylation factor (Arf) proteins are low molecular weight GTP binding proteins that belong to the Ras GTPase superfamily (1). Arf proteins are grouped into three distinct classes based on amino acid sequence and structural similarity, with Arf6 as the single class III protein to date. Arf6 is localized mainly to the plasma membrane and endosomes (1,2). This small GTPase interacts with PIP5K, PLD and Rac1, proteins important in lipid metabolism and actin regulation. Arf6 function depends upon its cycling between GDP- and GTP-bound states, which is regulated by associated GAP and GEF factors (3,4). Plasma membrane-associated Arf6 appears to play several functions during the many steps of membrane trafficking, including regulating membrane receptor internalization in both clathrin-dependent and independent pathways, endosomal recycling, and proximal actin reorganization and remodeling (5,6).