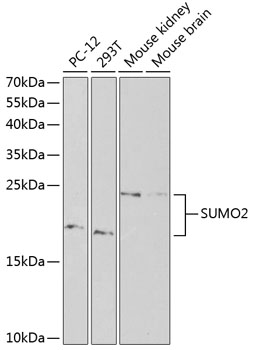
Small ubiquitin-related modifier 1, 2 and 3 (SUMO-1, -2 and -3) are members of the ubiquitin-like protein family (1). The covalent attachment of the SUMO-1, -2 or -3 (SUMOylation) to target proteins is analogous to ubiquitination. This post-translational modification is a reversible, multi-step process that is initiated by cleaving a precursor protein to a mature protein. Mature SUMO-1, -2 or -3 is then linked to the activating enzyme E1, conjugated to E2 and in conjunction with E3, SUMO-1, -2 or -3 is ligated to the target protein (2). Ubiquitin and the individual SUMO family members are all targeted to different proteins with diverse biological functions. Ubiquitin predominantly regulates degradation of its target (1). In contrast, SUMO-1 is conjugated to RanGAP, PML, p53 and IκB-α to regulate nuclear trafficking, formation of subnuclear structures, regulation of transcriptional activity and protein stability (3-7). SUMO-2/-3 forms poly-(SUMO) chains, is conjugated to topoisomerase II and APP, regulates chromosomal segregation and cellular responses to environmental stress, and plays a role in the progression of Alzheimer disease (8-11).