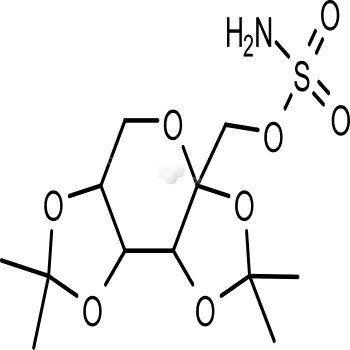
- Product NameTopiramate
- Brief DescriptionInhibitors
- Purification99.00%
- Biological ActivityTopiramate (brand name Topamax) is an anticonvulsant drug produced by Ortho-McNeil Neurologics, a division of Johnson & Johnson. It is used to treat epilepsy in both children and adults. In children it is also indicated for treatment of Lennox-Gastaut syndrome (a disorder that causes seizures and developmental delays). It is also Food and Drug Administration (FDA) approved for, and now most frequently prescribed for, the prevention of migraines. . A combination product containing phentermine and topiramate extended-release called QSYMIA? is indicated for the management of obesity. On August 2013, an extended released formulation, marketed as Trokendi XR has been approved for the management of partial onset, tonic-clonic, and Lennox-Gastaut Syndrome seizures.
- Target NameCarbonic Anhydrase inhibitor; GABA Receptor antagonist; GluR antagonist
- CAS No. 97240-79-4
- Calculated MW 339.36
- Formulation C12H21NO8S
- Storage 3 years -20˚C powder;2 years -80˚C in solvent;