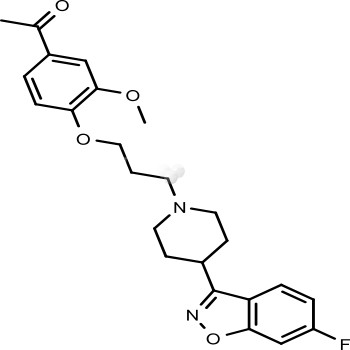
- Product NameIloperidone
- Brief DescriptionInhibitors
- Purification97.00%
- Biological ActivityIloperidone is an atypical antipsychotic for the treatment of schizophrenia symptoms. Hoechst Marion Roussel Inc. made initial inquiries into the drug; however, in May 1996, they discontinued research, and in June 1997 gave research rights to Titan Pharmaceuticals. Titan then handed over worldwide development, manufacturing and marketing rights to Novartis in August 1998. On June 9, 2004, Titan Pharmaceuticals announced that the Phase III development rights have been acquired by Vanda Pharmaceuticals. FDA approved on May 9, 2009.
- Target NameAdrenergic Receptor agonist; Dopamine Receptor antagonist
- CAS No. 133454-47-4
- Calculated MW 426.48
- Formulation C24H27FN2O4
- Storage 3 years -20˚C powder;2 years -80˚C in solvent;