- Product NameSN-38
- Brief DescriptionInhibitors
- Purification99.94%
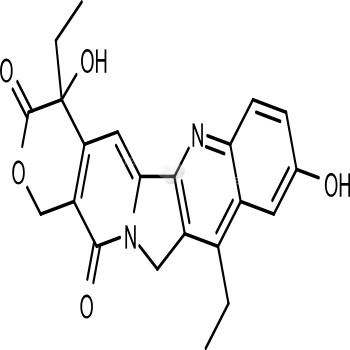
- Biological ActivityLE-SN38 is NeoPharms NeoLipid liposomal formulation of SN-38, the active metabolite of irinotecan (Camptosar), a chemotherapeutic pro-drug approved for the treatment of advanced colorectal Y. LE-SN38 is the Companys NeoLipid? Liposomal formulation of SN-38, the active, but poorly soluble, metabolite of Camptosar?, a chemotherapeutic pro-drug, which is used as a first-line and second-line treatment for advanced colorectal Y. A pro-drug is a compound that is converted into the active drug in the body. However, Camptosar? is converted into SN-38 in colorectal Y cells at different rates in different patients, and this variability in conversion rates may result in suboptimal treatment. By employing the Companys proprietary NeoLipid? technology to directly deliver SN-38, the Company hopes to minimize treatment variability. A Phase I clinical trial was completed in 2005 and showed the potential for decreased side effects, particularly diarrhea, compared to published results of Camptosar?. The results of that trial were presented at the American Society of Clinical Oncology (ASCO) meeting in June 2005, and were used to determine the Phase II study dose. .
- Target NameTopoisomerase inhibitor
- CAS No. 86639-52-3
- Calculated MW 392.4
- Formulation C22H20N2O5
- Storage 3 years -20˚C powder;2 years -80˚C in solvent;