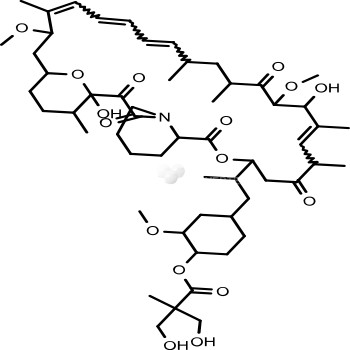
- Product NameTemsirolimus (CCI-779, NSC 683864)
- Brief DescriptionInhibitors
- Purification99.50%
- Biological ActivityTemsirolimus is an intravenous drug for the treatment of renal cell carcinoma (RCC), developed by Wyeth Pharmaceuticals and approved by the FDA in late May 2007, and was also approved by the European Medicines Agency (EMEA) on November 2007. It is a derivative of sirolimus and is sold as Torisel.
- Target NamemTOR inhibitor
- CAS No. 162635-04-3
- Calculated MW 1030.29
- Formulation C56H87NO16
- Storage 3 years -20˚C powder;2 years -80˚C in solvent;