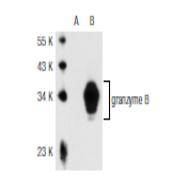
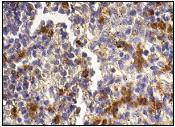
Granzyme A and granzyme B are serine proteases that mediate apoptotic signaling in cytotoxic T lymphocytes (CTL) and natural killer (NK) cells. Both granzyme A and granzyme B are synthesized as inactive proenzymes, and they are stored within cytolytic granules and released by effector cells during degranulation. In activated CTLs, granzyme A and granzyme B are processed and activated by cathepsin C, and they then function to induce apoptosis by two distinct pathways. Granzyme B proteolytically cleaves and activates members of the caspase family of cysteine proteases, including caspase-3, caspase-6, caspase-7 and caspase-9. When cleaved, these caspases assemble into active holoenzymes that then mediate apoptosis through a defined proteolytic cascade involving nuclear lamins and PARP (poly ADP ribose polymerase). Granzyme A mediates the activation of apoptosis by inducing single-strand DNA breaks, membrane perturbation and nuclear condensations in an alternative pathway that is independent from caspase activation or the caspase proteolytic cascade.