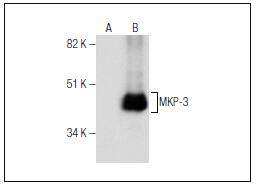
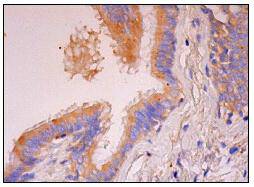
Mitogen-activated protein (MAP) kinases are a large class of proteins involved in signal transduction pathways that are activated by a range of stimuli and mediate a number of physiological and pathological changes in the cell. Dual specificity phosphatases (DSPs) are a subclass of the protein tyrosine phosphatase (PTP) gene superfamily, which are selective for dephosphorylating critical phosphothreonine and phosphotyrosine residues within MAP kinases. DSP gene expression is induced by a host of growth factors and/or cellular stresses, thereby negatively regulating MAP kinase superfamily members including MAPK/ERK, SAPK/JNK and p38. The members of the dual-specificity phosphatase protein family include MKP-1/CL100 (3CH134), VHR, PAC1, MKP-2, hVH-3 (B23), hVH-5, MKP-3, MKP-X, and MKP-4. Human MKP-3 maps to chromosome 12q21.33 and encodes a 381 amino acid protein that specifically inactivates members of the ERK family and is expressed in a variety of tissues with the highest levels in heart and pancreas.