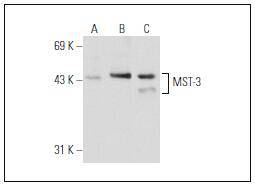
Sterile-20 (STE20) is a serine/threonine kinase in Saccharomyces cerevisiae that is involved in relaying signals from G protein-coupled receptors to cytosolic MAP kinase cascades. Mammalian protein kinases that display sequence similarity to STE20 are divided into two groups, the PAK subfamily and the GCK subfamily. The PAK subfamily members contain a C-terminal catalytic domain and an N-terminal regulatory domain with a p21Rac/Cdc42-binding site, and these kinases can activate both p38 MAPK and JNK. The GCK subfamily members contain a C-terminal regulatory domain and an N-terminal catalytic domain, and they have diverse roles in many pathways, including the activation of ERK, JNK, p38 MAPK, and caspase-3. The mammalian STE20-like kinases (MST kinases, also known as Ksr proteins) are members of the GCK subfamily. Ksr-1 and Ksr-2 (also known as MST-2 and MST-1, respectively) are both direct substrates of caspase-3 that accelerate caspase-3 activation. MST-3 is ubiquitously expressed in mammalian tissue and can phosphorylate exogenous substrates as well as itself. MST-4 is highly expressed in placenta, thymus, and peripheral blood leukocytes, and it specifically activates ERK.