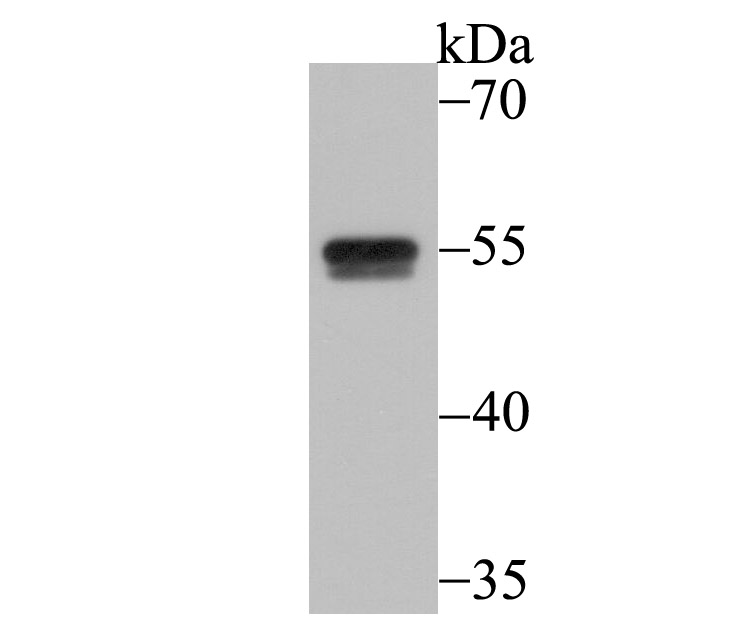
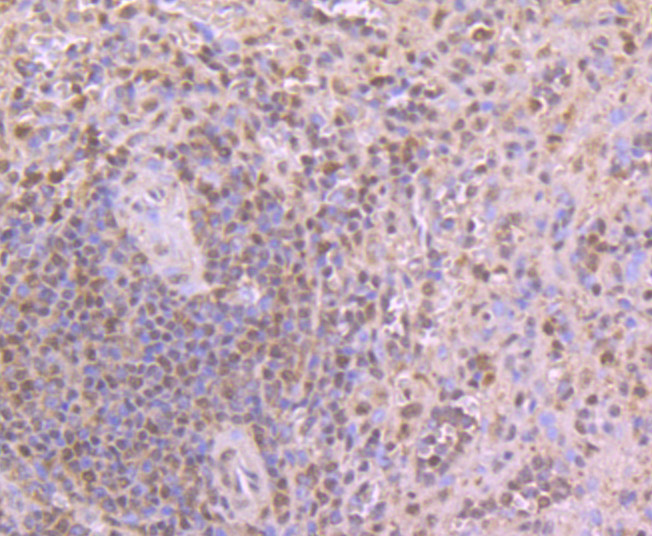
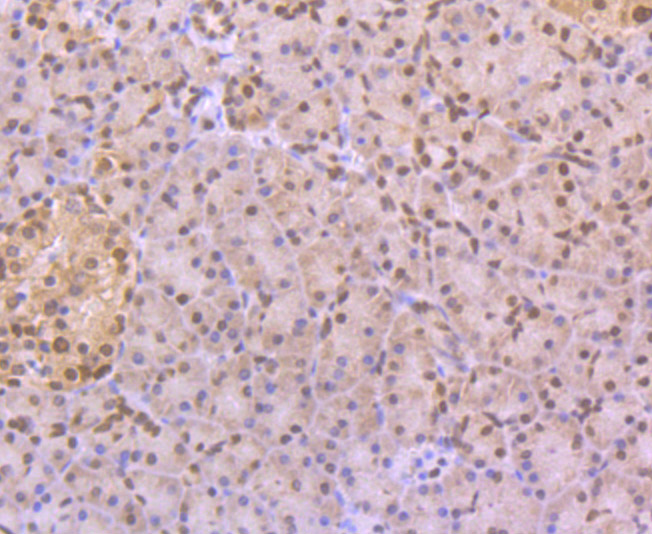
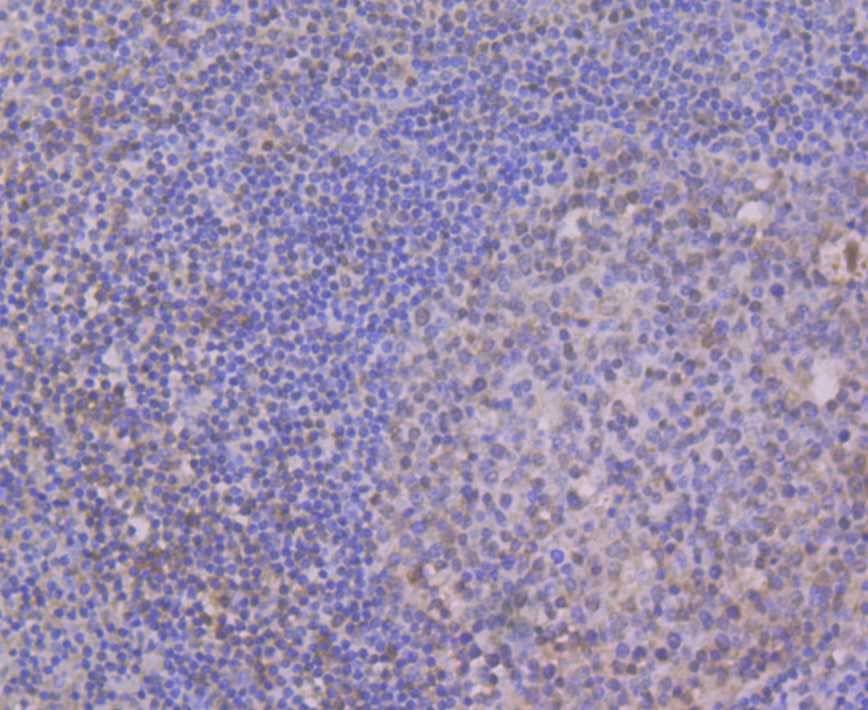
Myotonic dystrophy (DM) is an autosomal dominant neuromuscular disease that is associated with a (CTG)n repeat expansion in the 3'-untranslated region of the myotonin protein kinase gene (DMPK). CUG-BP1 and CUG-BP2 are proteins that bind specifically to (CUG)8 oligonucleotides in vitro. While CUG-BP1 has the major binding activity in normal cells, nuclear CUG-BP2 binding activity increases in DM cells. Both CUG-BP1 and CUG-BP2 are isoforms of a novel heterogeneous nuclear ribonucleoprotein (hnRNP), hNab50. CUG-BP1, an RNA CUG triplet repeat binding protein, regulates splicing and translation of various RNAs. Expansion of RNA CUG repeats in the DMPK in DM is associated with alterations in binding activity of CUG-BP1 as well as alterations in the translation of the C/EBPb transcription factor. CUG-BP1 is an important regulator of initiation from different AUG codons of C/EBPb mRNA. In normal cells, CUG-BP1 up-regulates the p21 protein during differentiation by inducing the translation of p21 via binding to a GC-rich sequence located within the 5' region of p21 mRNA. In DM cells, failure to accumulate CUG-BP1 leads to a reduction of p21 and alterations in other proteins responsible for cell cycle withdrawl.