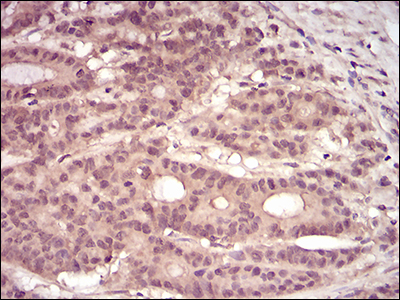
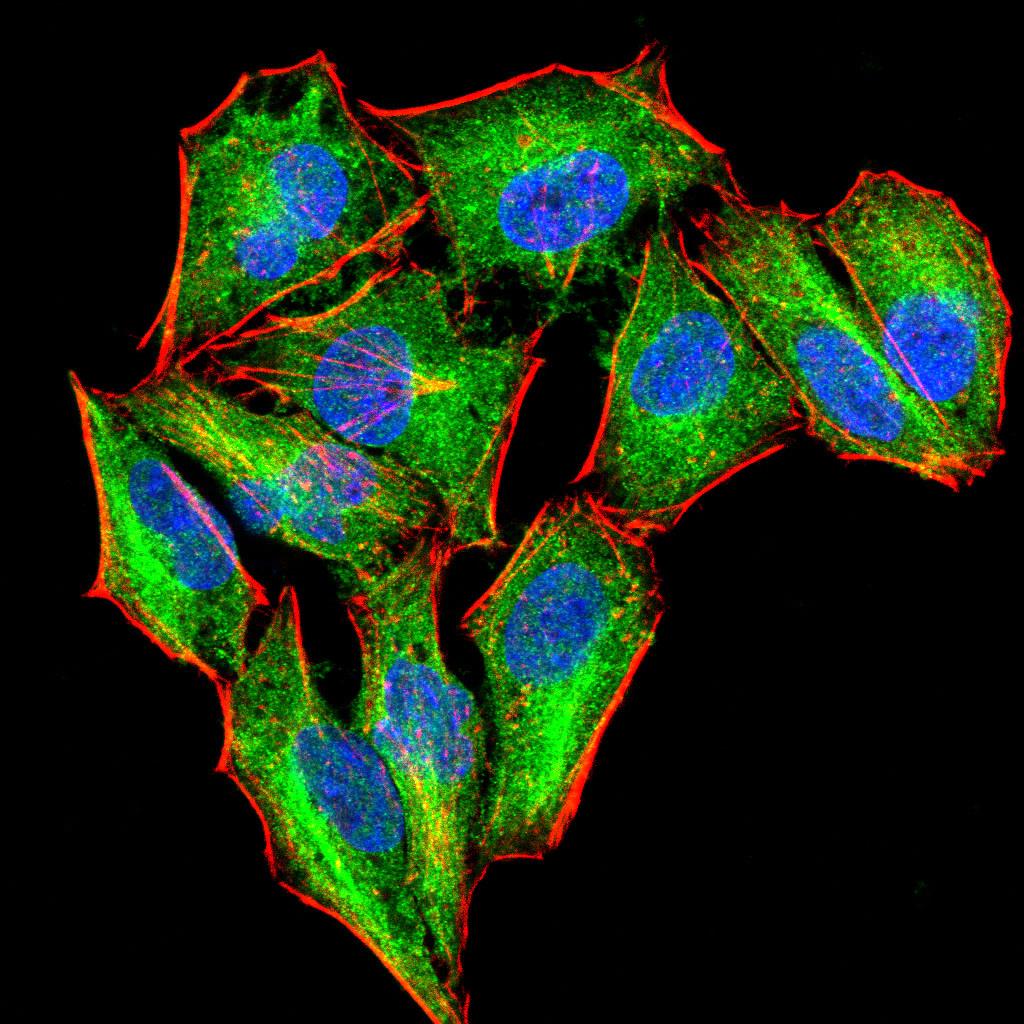
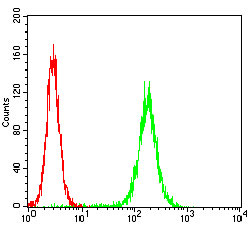
Rho, the Ras-related small GTPase, is responsible for the regulation of actin-based cytoskeletal structures including stress fibers, focal adhesions and the contractile ring apparatus. Rho proteins act as molecular switches which are able to turn cytokinesis on and off. Although little is know about signaling downstream of Rho, several proteins have been implicated as Rho effectors. Protein kinase N (PKN) is a fatty acid-activated serine/threonine kinase whose catalytic domain exhibits homology with that of the PKC family. PKN associates with Rho via its amino terminus, is activated in a GTP-dependent manner and phosphorylates the head-rod domain of neurofilament protein. A second protein, rhophilin, exhibits 40% sequence identity with the amino terminal Rho binding domain. The enzymatic activity of rhophilin has not been demonstrated and it is possible that it acts through the recruitment of cytoskeletal components that initiate a kinase signaling cascade. Citron interacts specifically with active Rho and Rac1 but not Cdc42. Citron exhibits a distinctive protein organization and little homology with the Rho binding domains of PKN and rhophilin.