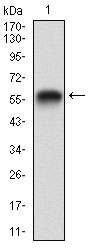
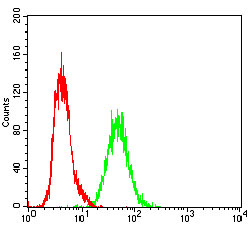
Rap1 GTPase activating protein (Rap1GAP) specifically stimulates GTP hydrolytic activity of the monomeric G protein Rap1. Physical interaction between Gα z, a member of the Gi family of trimeric G proteins, and Rap1GAP blocks the ability of regulators of G protein signaling to stimulate GTP hydrolysis of the α subunit, and also attenuates the ability of activated Gα z to inhibit adenylyl cyclase. Rap1GAP is expressed in the brain, kidney and pancreas and may act as a signal integrator to coordinate and/or integrate Gz signaling and Rap1 signaling in cells. A novel isoform of Rapl GTPase-activating protein, designated Rap1GAPII, binds specifically to Gα z. Stimulation of the Gi-coupled M2 Muscarinic receptor translocates Rap1GAPII from the cytosol to the membrane and decreases the amount of GTP-bound Rap1, resulting in the activation of ERK/MAPK.