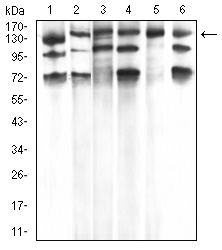
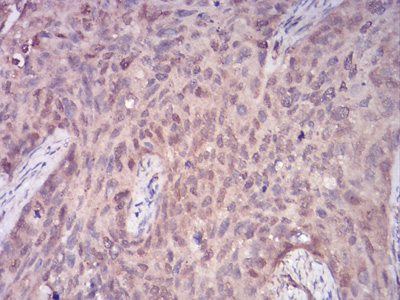
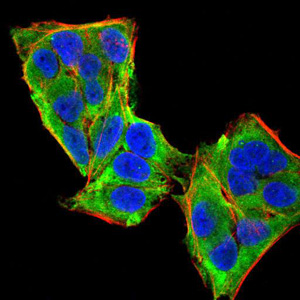
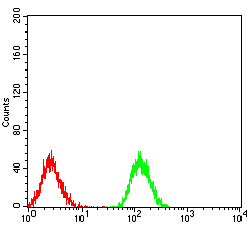
Phospholipase C-gamma 1 (PLC g1) is an isozyme of the phosphoinositide-specific PLC family, which occupies a central role in hormonal signal transduction pathways and is a substrate for the epidermal growth factor receptor tyrosine kinase. Following activation of TrkB, PLC g1 is phosphorylated on Tyrosine 783, Tyrosine 771 and Tyrosine 1253. Tyrosine 783 lies just downstream of the kinase domain in a relatively short sequence motif characteristic of the Trk family of protein-tyrosine kinase receptors. The sequence around Tyrosine 783 fits a consensus sequence for binding PLC g1. PLC g1 also forms a complex with TrkB consistent with the possibility that one of the TrkB autophosphorylation sites provides a binding site for the PLC g1 SH2 domains, as is the case for other receptor protein-tyrosine kinases.