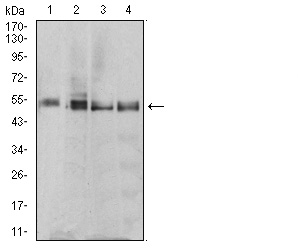
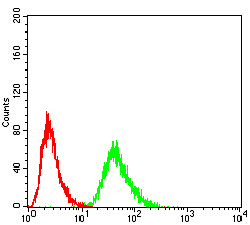
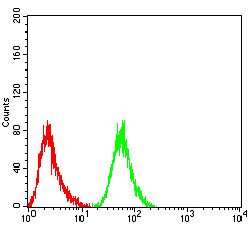
Translation is carried out by the ribosome and several associated protein factors through three consecutive steps: initiation, elongation and termination. Termination of protein synthesis takes place when the ribosomal A site is occupied simultaneously by one of three stop codons and by a class 1 translation termination factor. In eukaryotes, this termination factor is the eukaryotic release factor 1 (eRF1), a protein that promotes hydrolysis of the last peptidyl-tRNA on the ribosome. eRF1 activity is stimulated by the association with the GTP-binding protein eRF3. eRF1 forms a quaternary complex with eRF3, GTP and the ribosome. This complex performs a dual role, where, in the "GTP state," it controls the positioning of eRF1 toward the stop codon and peptidyl-tRNA, and, in the "GDP state," it promotes the release of the eRFs from the ribosome. eRF1 contains a highly conserved Asn-Ile-Lys-Ser (NIKS) tetrapeptide, which is essential for the interaction of eRF1 with the ribosome. The gene encoding human eRF1 maps to chromosome 5q31.2.