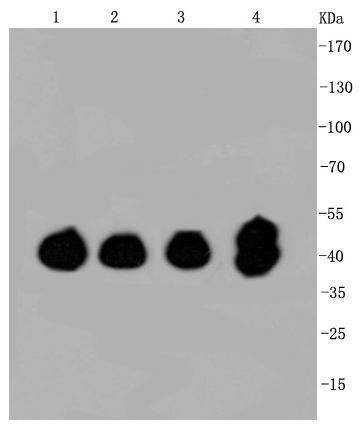
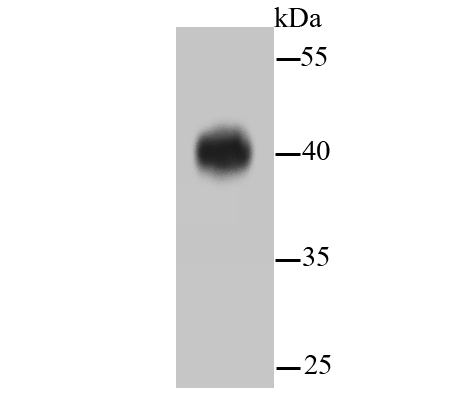
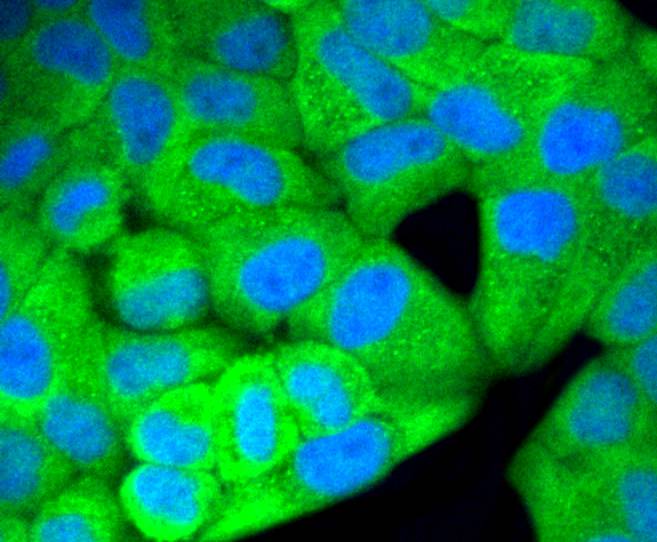
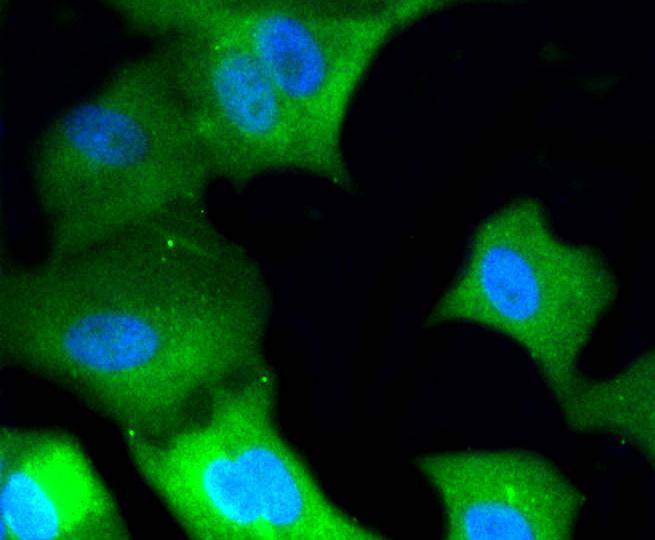
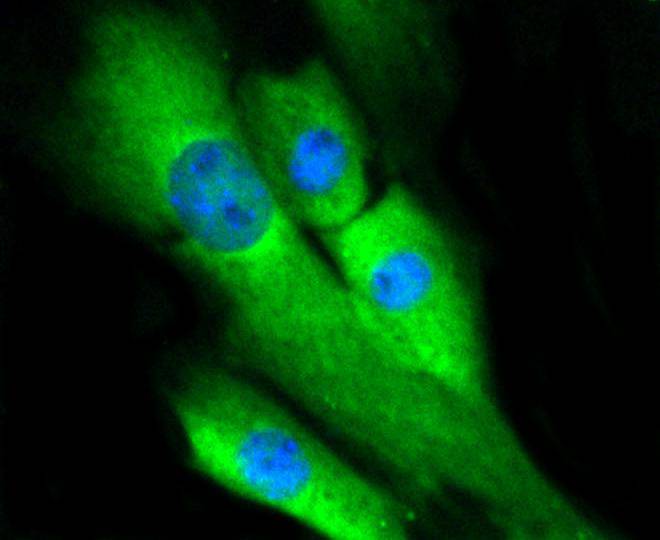
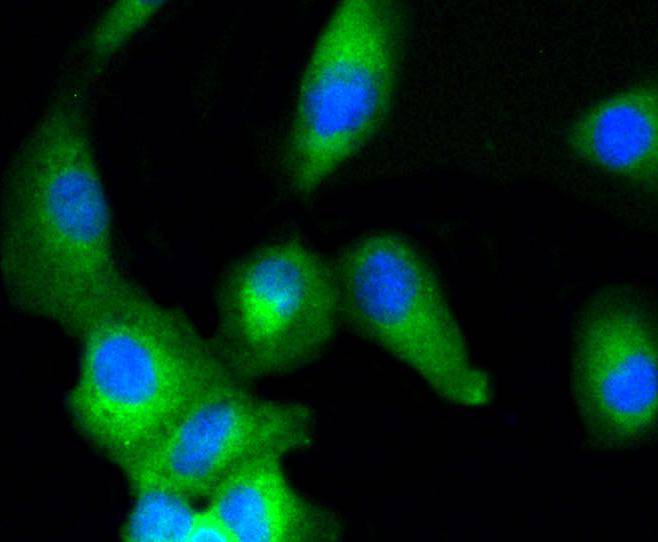
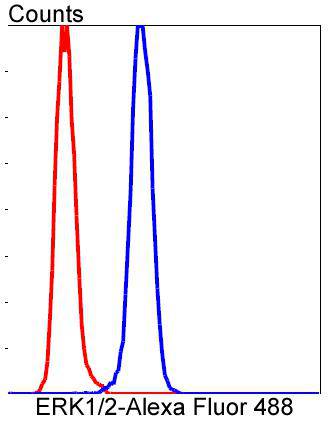
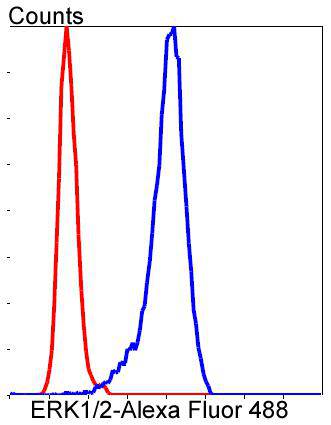
Mitogen-activated protein kinase (MAPK) signaling pathways involve two closely related MAP kinases, known as extracellular-signal-related kinase 1 (ERK 1, p44) and 2 (ERK 2, p42). Growth factors, steroid hormones, G protein-coupled receptor ligands and neurotransmitters can initiate MAPK signaling pathways. Activation of ERK 1 and ERK 2 requires phosphorylation by upstream kinases such as MAP kinasekinase (MEK), MEK kinase and Raf-1. ERK 1 and ERK 2 phosphorylation can occur at specific tyrosine and threonine sites mapping within consensus motifs that include the threonine-glutamate-tyrosine motif. ERK activation leads to dimerization with other ERKs and subsequent localization to the nucleus. Active ERK dimers phosphorylate serine and threonine residues on nuclear proteins and influence a host of responses that include proliferation, differentiation, transcription regulation and development. The human ERK 1 gene maps to chromosome 16p11.2 and encodes a 379 amino acid protein that shares 83% sequence identity to ERK 2. The human ERK2 gene maps to chromosome 22q11.21 and encodes a 360-amino acid protein.