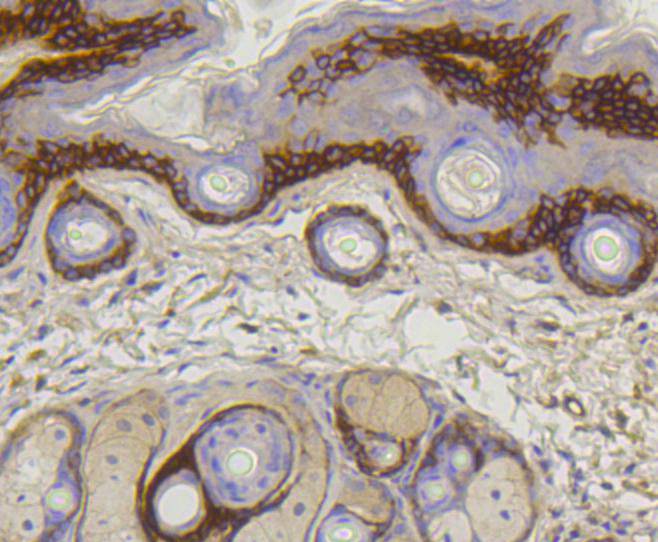
The extensive collagen family comprises several chain types, including fibril-forming interstitial collagens and basement membrane collagens, with each type containing multiple isoforms. Products of the COL gene family, collagens are characterized as fibrous, extracellular matrix proteins with high tensile strength that constitute the major components of connective tissues, such as tendons and cartilage. All collagens contain a triple helix domain and frequently show lateral self-association in order to form complex connective tissues. Collagen Type XVII, also designated BP180, represents a type II transmembrane, epithelial adhesion molecule that plays a role in cell migration and differentiation. The full length Collagen Type XVII protein is expressed in hemidesmosomes of keratinocytes. Proteolytic shedding of Collagen Type XVII results in a species in the extracellular matrix, and this process may be mediated by a disintegrin and metalloprotease (ADAM) family member. The BPAG2 gene, which encodes the Collagen Type XVII protein, maps to human chromosome 10q25.1. Mutations in this gene result in Bullous pemphigoid, an inflammatory subepidermal blistering skin disease associated with an IgG autoimmune response to Collagen Type XVII.