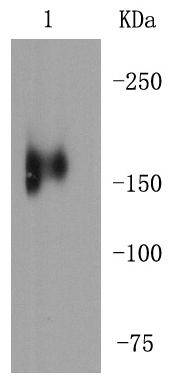
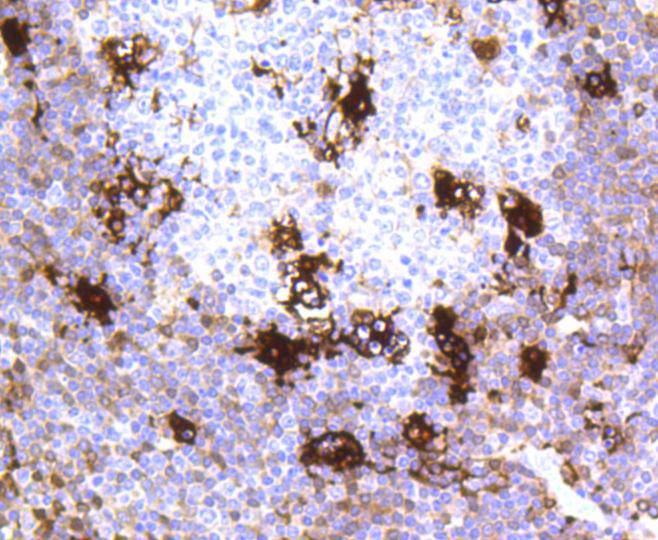
MerTK, also called c-Mer, is a member of the Mer/Axl/Tyro3 receptor kinase family. It is a 984 residue transmembrane protein made up of one tyrosine kinase domain, two Fibronectin type-III domains and two immunoglobulin-like C2-type domains. MerTK is the mammalian ortholog of the chicken retroviral oncogene product v-Eyk. This protein plays a critical role in macrophage activation, platelet aggregation, clot stability and the efficient removal of apoptotic cells. Specifically, MerTK acts as a signaling molecule, triggering outer segment ingestion in the retinal pigment epithelium (RPE) phagocytic process. Evidence suggests that MerTK signals via interaction with phosphatidylinositol-specific phospholipase C 2 (PI-PLC 2). When the gene encoding for MerTK is mutated, the RPE phagocytosis pathway is disrupted and autosomal recessive retinitis pigmentosa (RP) may result, leading to degeneration of retinal photoreceptor cells.