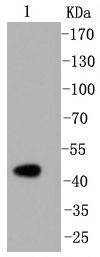
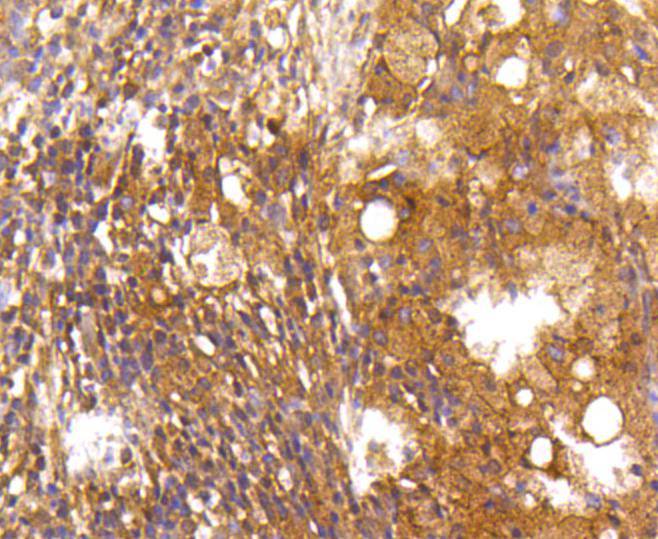
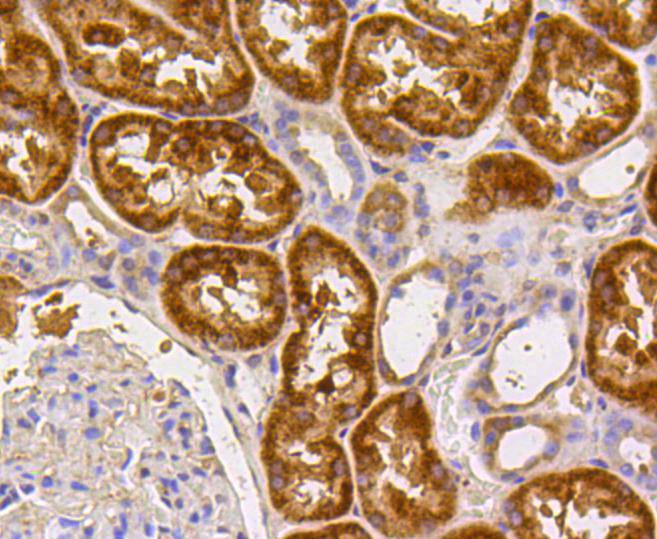
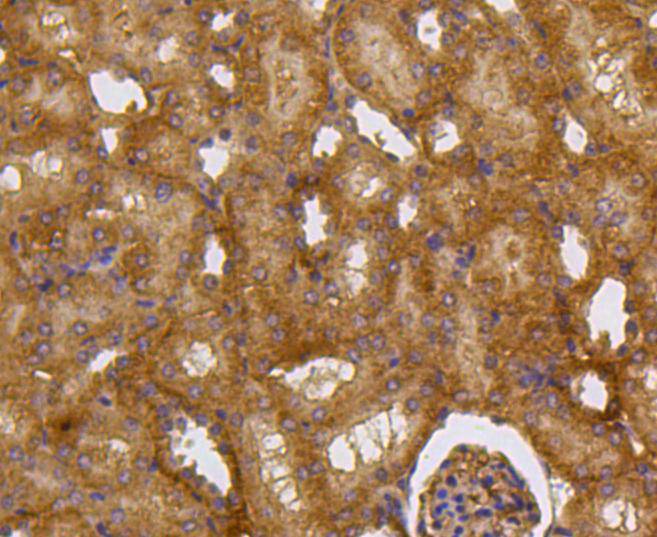
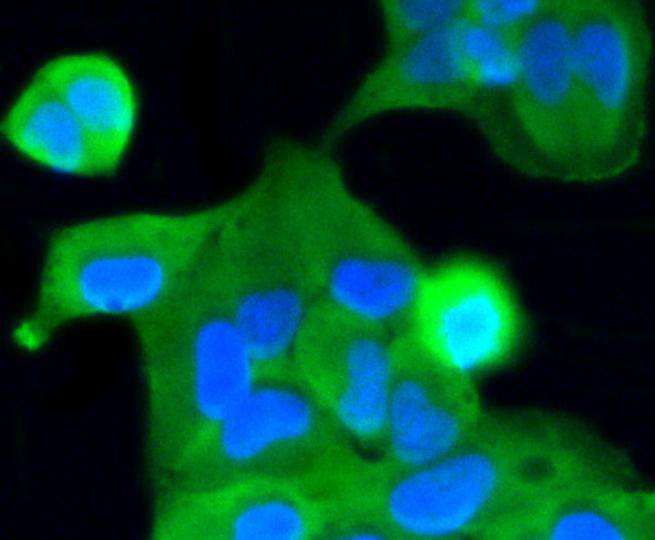
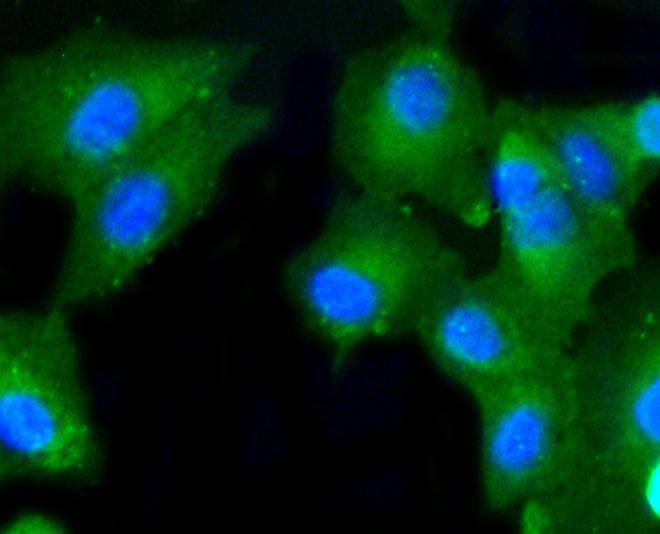
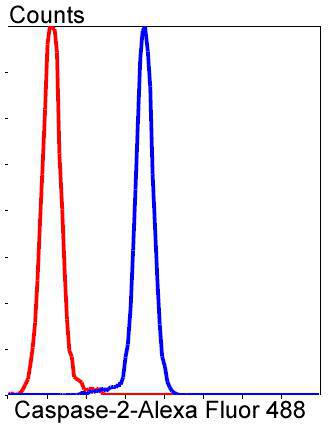
Caspase-2 (Nedd2, ICH-1) is an aspartate-specific cysteine protease that is activated in response to various apoptotic stimuli. Caspase-2 is unique among the caspases in that it has features of both upstream caspases (long prodomain) and downstream caspases (DEXD substrate specificity). Caspase-2 is highly expressed in the brain during development, and is expressed at low levels in adult tissue. Specifically, caspase-2 localizes to the mitochondria, the Golgi, the cytoplasm, and the nucleus. Caspase-2 exists as two isoforms, caspase-2L and caspase-2S, which are produced by alternative splicing and differ in their N and C-termini. Caspase-2L acts as a positive regulator of apoptosis, whereas caspase-2S functions as a negative regulator of apoptosis. Following apoptotic stimuli, the caspase-2L precursor undergoes cleavage at Asp-153 to produce a fragment (p30). The p30 fragment undergoes further cleavage to generate a fragment containing amino acids 153-308 (p18) and a fragment containing amino acids 317-435 (p13 or p14). As apoptosis progresses, the p13 (p14) fragment can undergo further processing to yield a fragment containing amino acids 331-435 (p12).