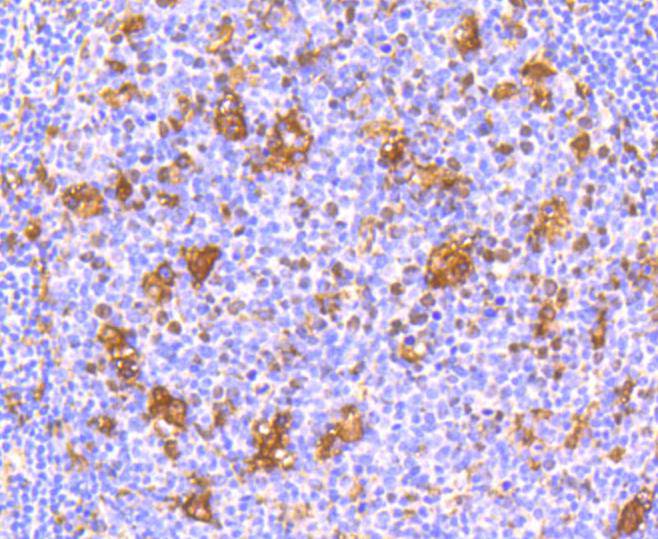
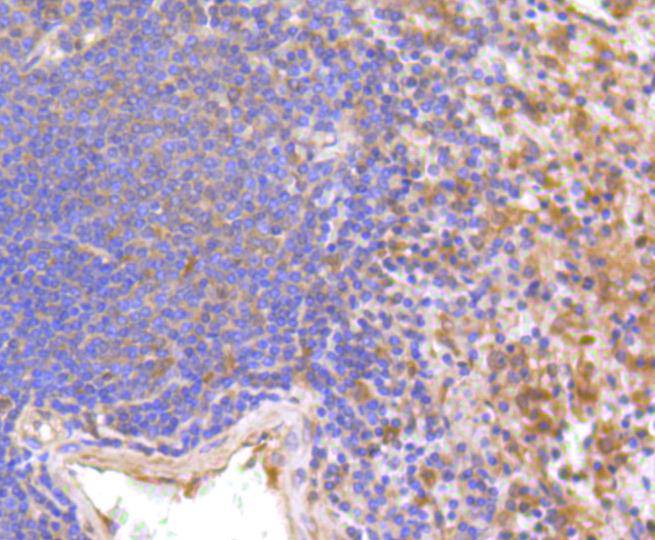
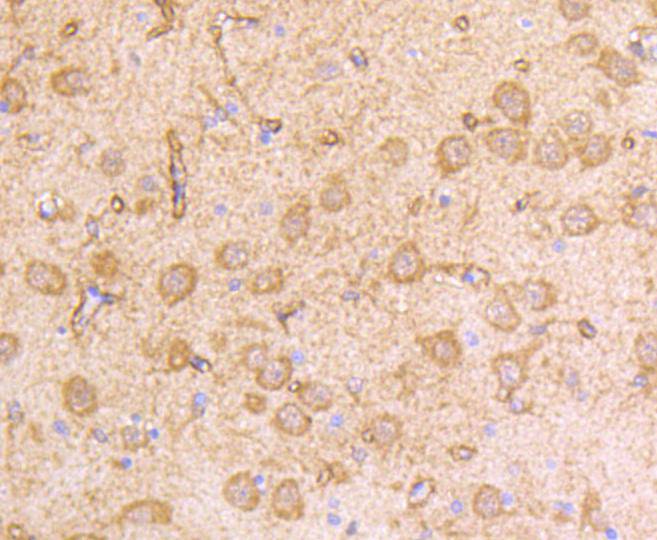
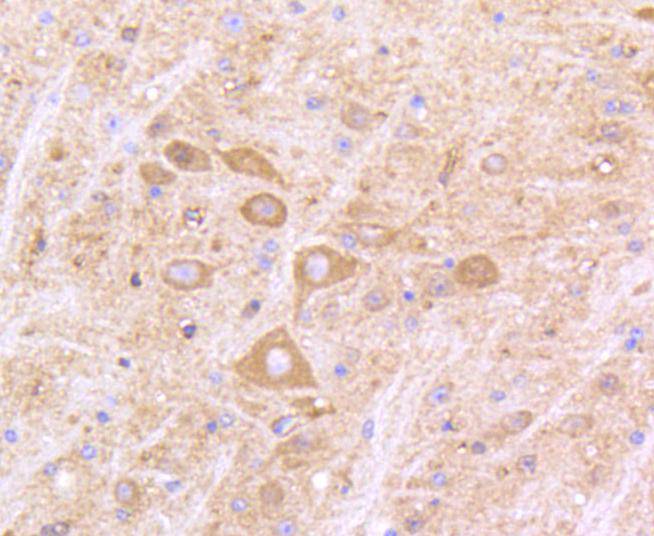
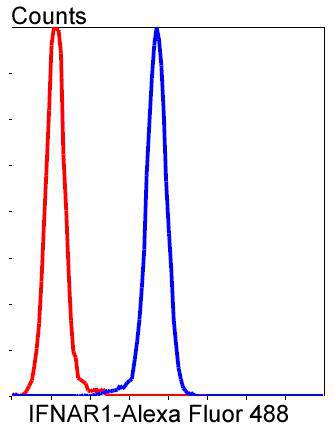
The type I interferons (IFNs), α and β, are a group of structurally and functionally related proteins that are induced by either viruses or double stranded RNA and defined by their ability to confer an antiviral state in cells. The α and β IFNs appear to compete with one another for binding to a common cell surface receptor, while immune IFN (IFNγ) binds to a distinct receptor. The latter protein, IFN-αR, is only weakly responsive to type I interferons in contrast to IFN-α/βR, which binds to and responds effectively to IFN-β and to several of the IFN-? subtypes. Moreover, IFN-α/βR is physically associated with the cytoplasmic tyrosine kinase JAK1 and thus, in addition to ligand binding, appears to be functionally involved in signal transduction. IFN-αR1 is a receptor for IFN-α/β and is present as the full chain (IFN-αR1a) and as a splice-variant (IFN-αR1). The IFN-γ receptor complex consists of an alpha subunit (IFN-γRα) and a beta subunit that is 332 amino acids in length (mouse) and 337 amino acids in length (human).