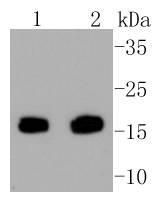
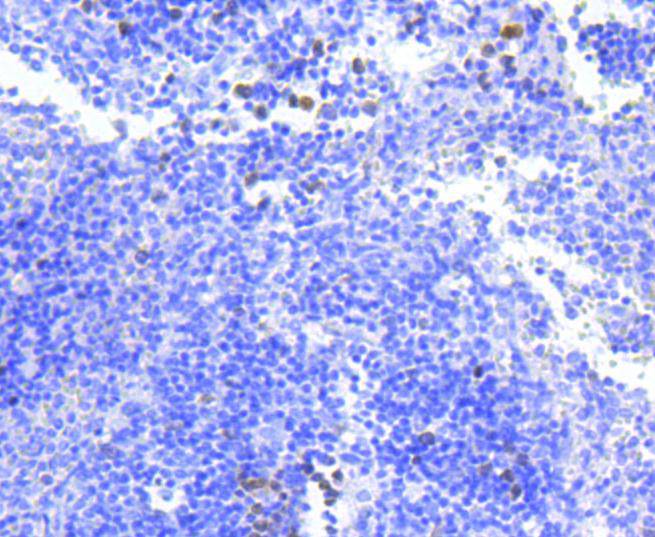
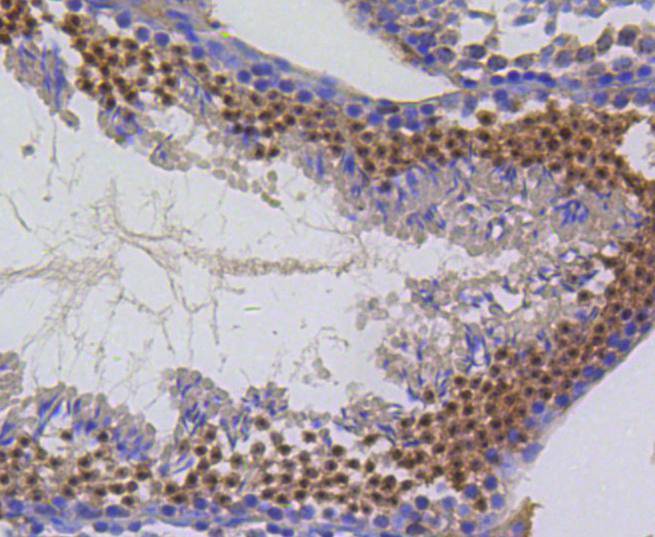
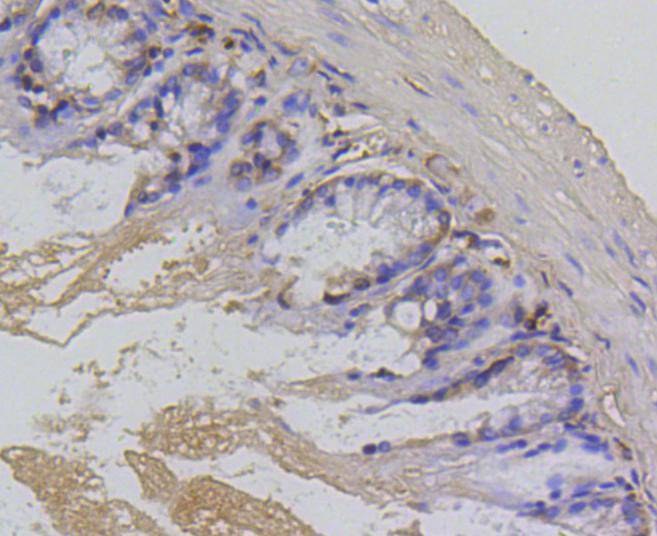
The baculovirus protein p35 inhibits virally-induced apoptosis of invertebrate and mammalian cells and may function to impair the clearing of virally infected cells by the immune system of the host. This is accomplished at least in part by the ability of p35 to block both TNF- and FAS-mediated apoptosis through the inhibition of the ICE family of serine proteases. Two mammalian homologs of baculovirus p35, referred to as inhibitor of apoptosis protein (IAP) 1 and 2, share an amino-terminal baculovirus IAP repeat (BIR) motif and a carboxy-terminal RING finger. Although the c-IAPs do not directly associate with the TNF receptor (TNF-R), they efficiently block TNF-mediated apoptosis through their interaction with the downstream TNF-R effectors, TRAF1 and TRAF2. Additional IAP family members include ILP (for IAP-like protein) and survivin. ILP inhibits activated caspase-3, leading to the resistance of FAS-mediated apoptosis. Survivin (also designated TIAP) is expressed during the G2/M phase of the cell cycle and associates with microtubules of the mitotic spindle. Increased caspase-3 activity is detected when a disruption of survivin-microtubule interactions occurs.