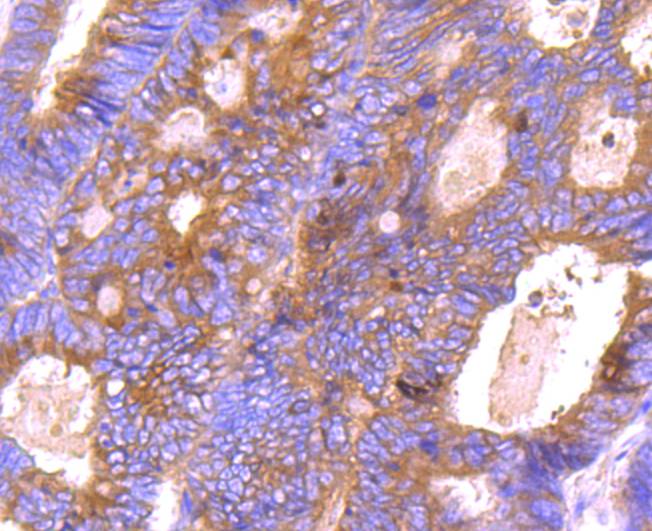
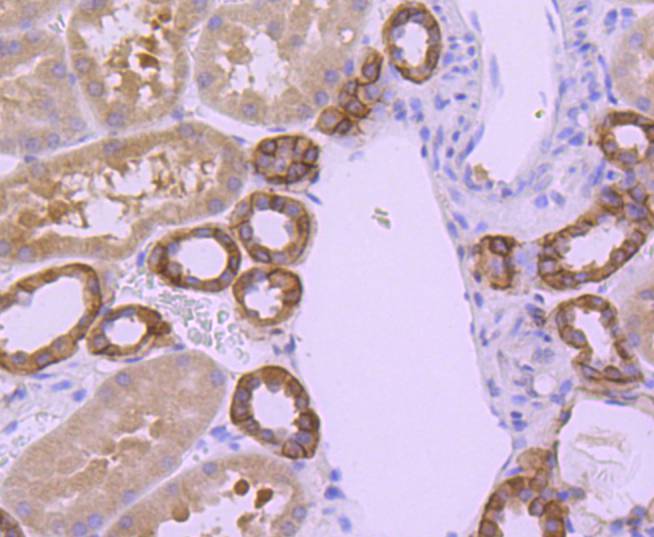
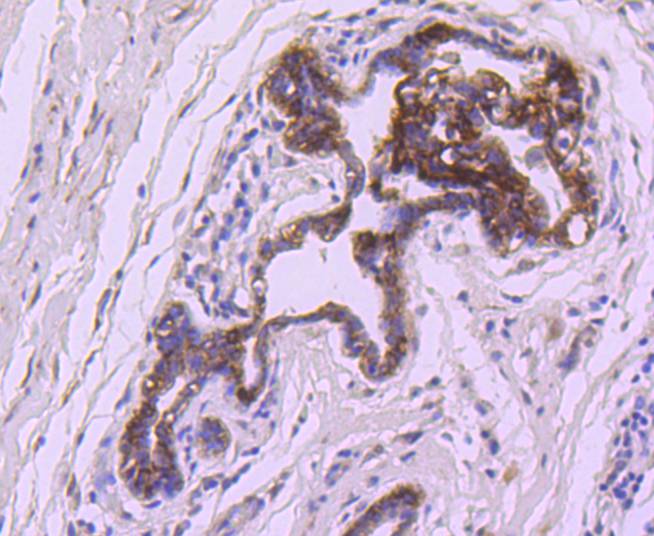
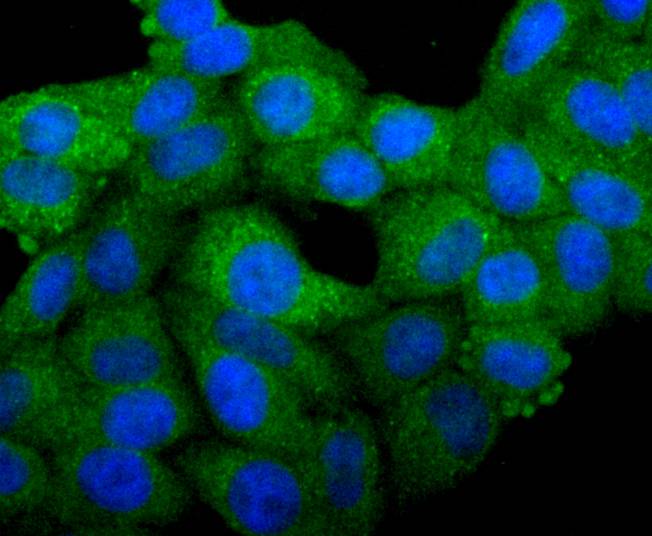
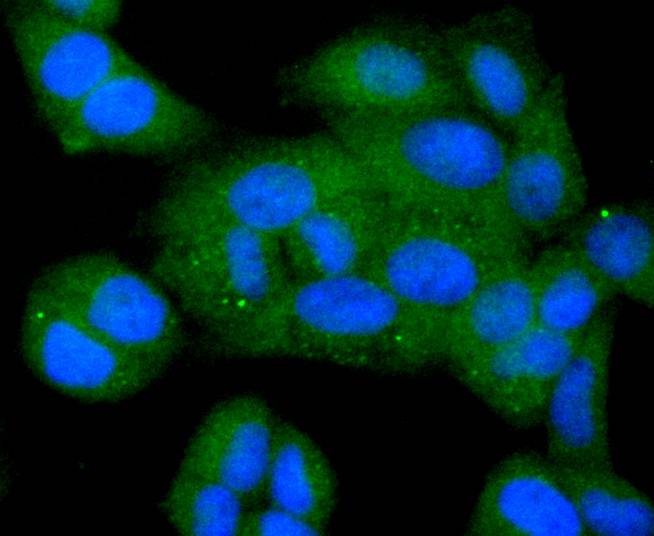
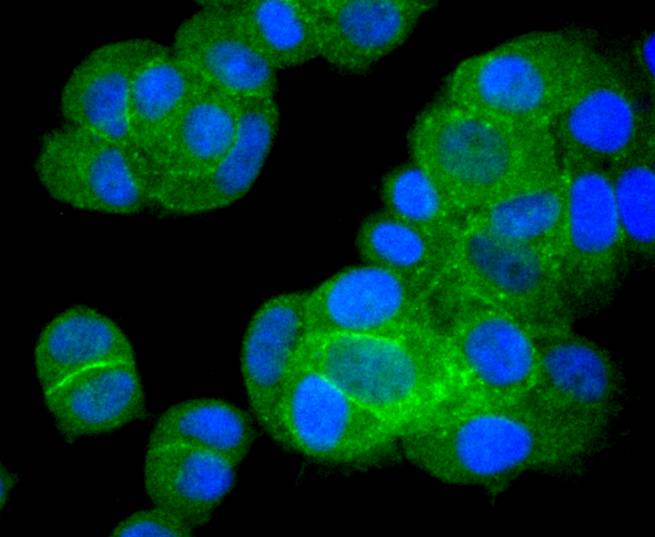
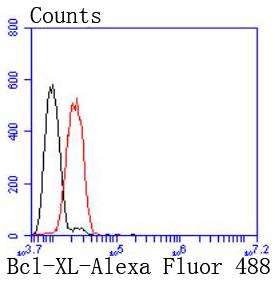
The Bcl-2 gene was isolated at the chromosomal breakpoint of t(14;18) bearing follicular B cell lymphomas. Bcl-2 blocks cell death following a variety of stimuli and confers a death-sparing effect to certain hematopoietic cell lines following growth factor withdrawal. A second protein, designated Bcl-associated X protein (Bax) p21, has extensive amino acid homology with Bcl-2 and both homodimerizes and heterodimerizes with Bcl-2. Overexpression of Bax accelerates apoptotic death induced by cytokine deprivation in an IL-3-dependent cell line, and Bax also counters the death repressor activity of Bcl-2. Bcl-x, one of several additional proteins with sequence homology to Bcl-2, is expressed as Bcl-xL, a 233 amino acid protein with 43% sequence identity with Bcl-2 that suppresses cell death, and Bcl-xS, a shorter variant that is 178 amino acids in length and lacks a 63 amino acid region (amino acids 126-188) found in Bcl-xL and which functions as a dominant inhibitor of Bcl-2. A further apoptosis-inducing protein, Bad, dimerizes both with Bcl-xL and to a lesser extent with Bcl-2, thus displacing Bax and inducing apoptosis.