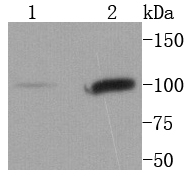
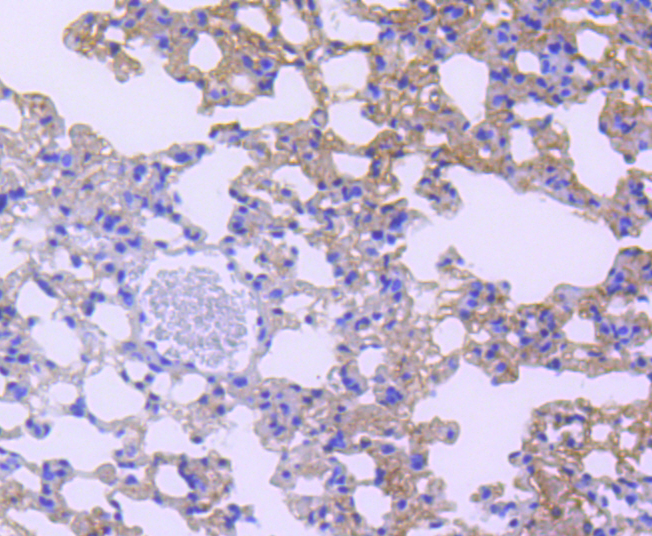
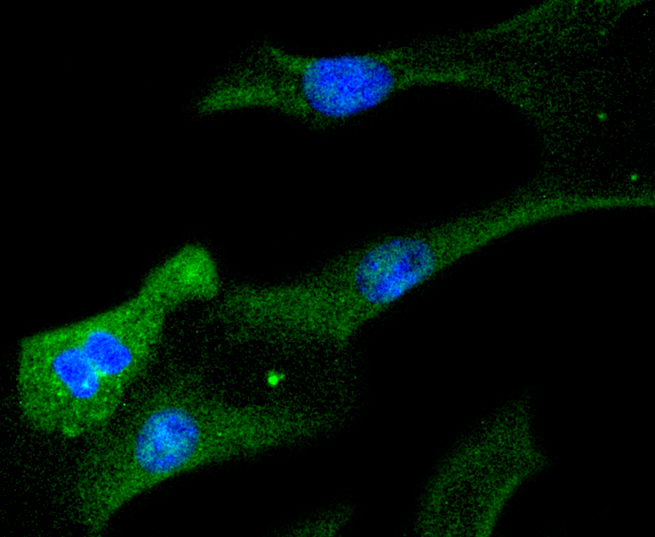
Membrane receptor signaling by various ligands, including interferons and growth hormones such as EGF, induces activation of JAK kinases which then leads to tyrosine phosphorylation of proteins that have been designated Stats (signal transducers and activators of transcription). The first members of this family to be described include Stat1α p91, Stat1β p84 (a form of p91 that lacks 38 COOH-terminal amino acids) and Stat2 p113. Stat1 and Stat2 are induced by IFN-a and form a heterodimer which is part of the ISGF3 transcription factor complex. Stat3, which becomes activated in response to epidermal growth factor (EGF) and interleukin-6 (IL-6), but not interferon-γ (IFN-γ) or Stat4, is an additional member of this family. It has been suggested that the phosphorylated forms of both Stat3 and Stat4 form homodimers as well as heterodimers with the other members of the Stat family, and that differential activation of different Stat proteins in response to different ligands should help to explain specificity in nuclear signaling from the cell surface. Highest expression of Stat4 is seen in testis and myeloid cells. IL-12 has been identified as an activator of Stat4. Other members of the Stat family include Stat5, which has been shown to be activated by prolactin and by IL-3, and Stat6 (also designated IL-4 Stat), which is involved in IL-4-activated signaling pathways.