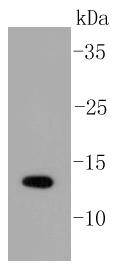
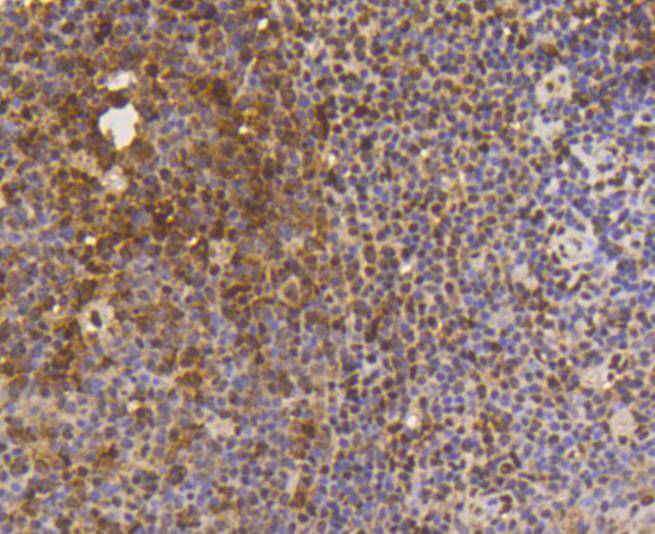
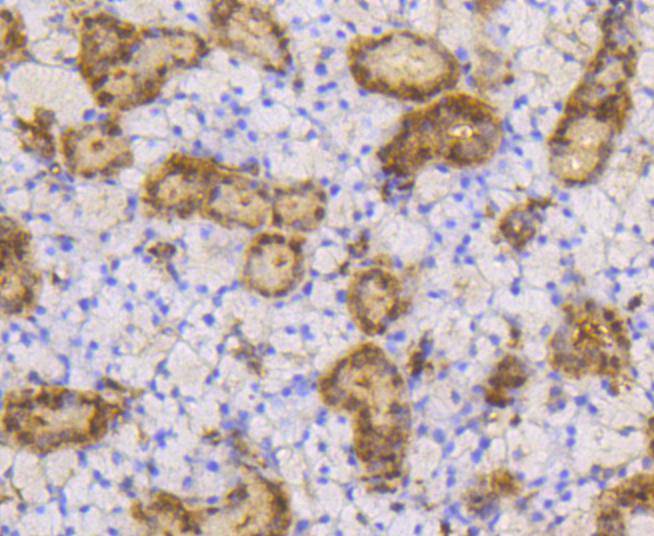
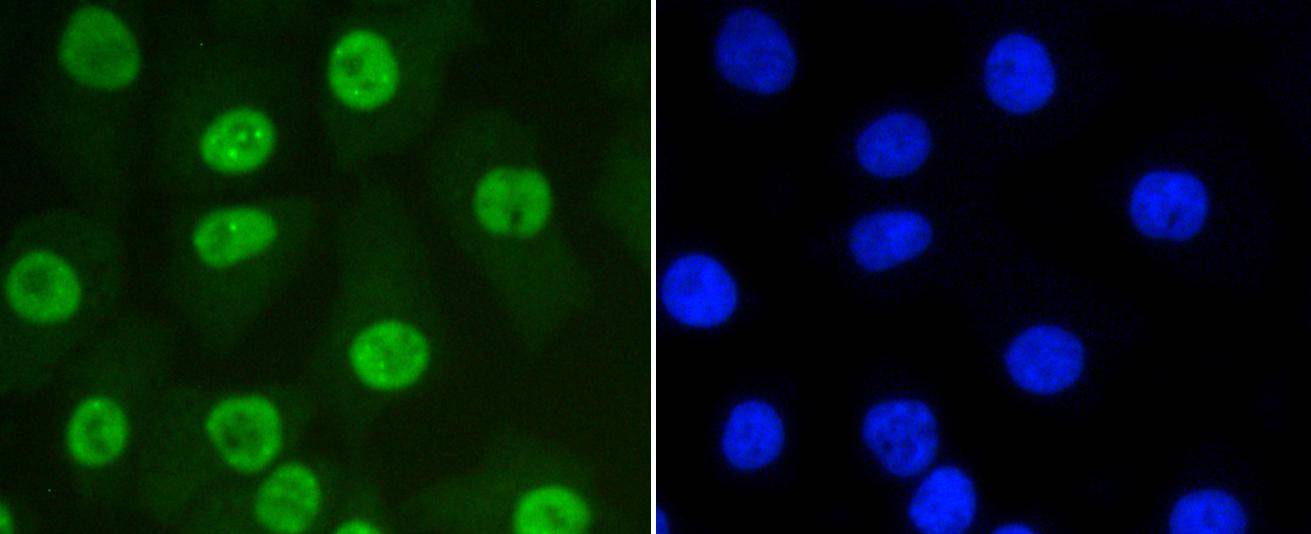
The small ubiquitin-related modifier (SUMO) proteins, which include SUMO-1, SUMO-2 and SUMO-3, belong to the ubiquitin-like protein family. Like ubiquitin, the SUMO proteins are synthesized as precursor proteins that undergo processing before conjugation to target proteins. Also, both utilize the E1, E2, and E3 cascade enzymes for conjugation. However, SUMO and ubiquitin differ with respect to targeting. Ubiquitination predominantly targets proteins for degradation, whereas sumoylation targets proteins to a variety of cellular processing, including nuclear transport, transcriptional regulation, apoptosis and protein stability. The unconjugated SUMO-1, SUMO-2 and SUMO-3 proteins localize to the nuclear membrane, nuclear bodies and cytoplasm, respectively. SUMO-1 utilizes Ubc9 for conjugation to several target proteins, which include IkBa, MDM2, p53, PML and Ran GAP1. SUMO-2 and SUMO-3 contribute to a greater percentage of protein modification than does SUMO-1, and unlike SUMO-1, they can form polymeric chains. In addition, SUMO-3 regulates b-Amyloid generation and may be critical in the onset or progression of Alzheimers disease.