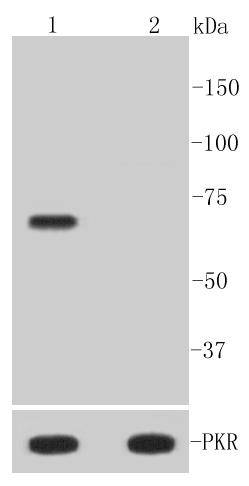
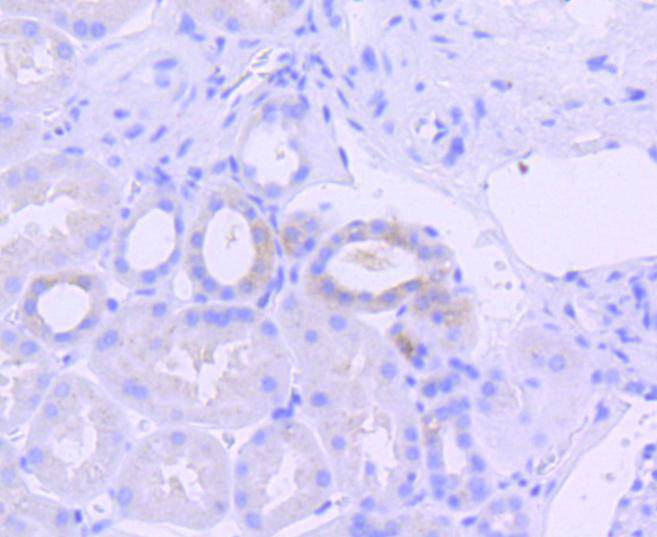
An interferon-inducible, RNA-dependent protein serine/threonine kinase, PKR has various designations. Mouse PKR is known as DAI, dsJ, PI kinase, p65, p67 or TIK, whereas human PKR is known as p68 or p69. PKR phosphorylates its substrate, a subunit of protein synthesis initiation factor eIF-2 on Ser 51 to inhibit translation. PKR contains two dsRNA binding motifs required for its activation by dsRNA. Three kinds of regulation of PKR enzymatic activity occur, and these include transcriptional regulation in response to interferon, an autoregulatory mechanism controlling PKR expression at the level of translation, and posttranslational regulation by RNA mediated autophosphorylation. Human PKR contains at least 15 autophosphorylation sites, but only Thr-446 and Thr-451 in the activation loop are critical for its kinase activity. Thr-446 is the in vivo autophosphorylation site of PKR. Mutation of threonine to alanine at position 446 substantially reduces PKR function, and mutant kinase containing Ala-451 is completely inactive.