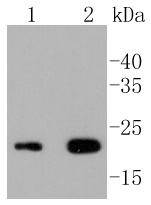
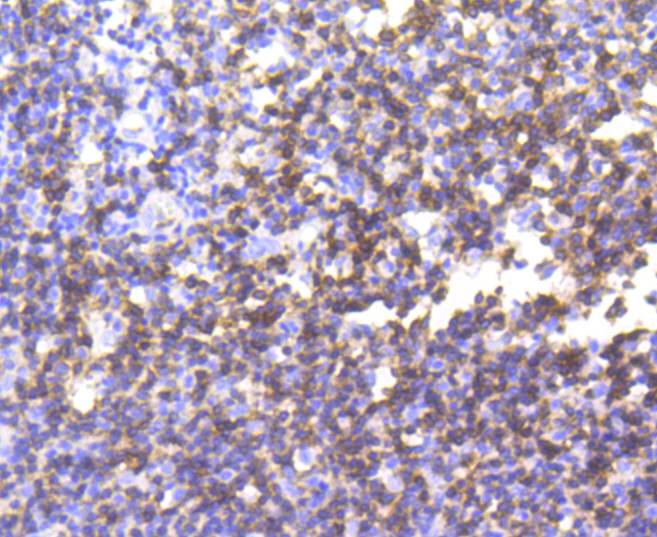
The T cell antigen receptor (TCR) recognizes foreign antigens and translates such recognition events into intracellular signals that elicit a change in the cell from a dormant to an activated state. Much of this signaling process can be attributed to a multisubunit complex of proteins that associates directly with the TCR. This complex has been designated CD3 (cluster of differentiation 3). CD3 is composed of four polypeptides: ζ, γ, ε and δ. Each of these polypeptides contains at least one immunoreceptor tyrosine-based activation motif (ITAM). Engagement of TCR complex with foreign antigens induces tyrosine phosphorylation in the ITAM motifs and phosphorylated ITAMs function as docking sites for signaling molecules such as ZAP-70 and p85 subunit of PI-3 kinase. TCR ligation also induces a conformational change in CD3ε, such that a proline region is exposed and then associates with the adaptor protein Nck.