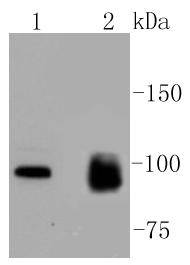
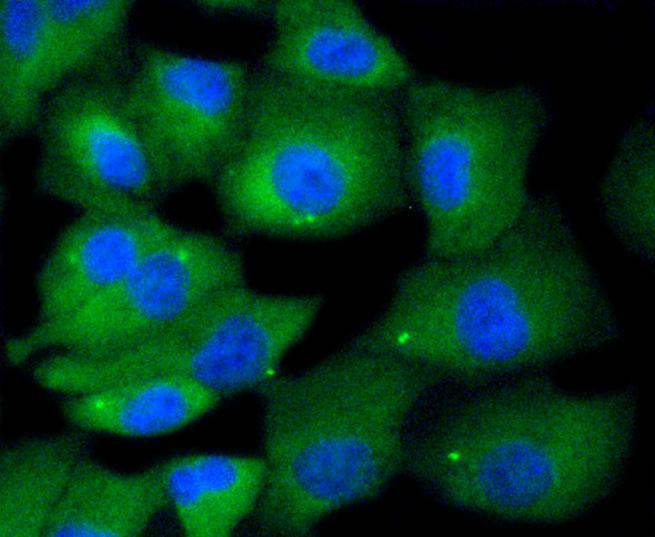
The monoclonal antibody CHO1 detects a spindle antigen required for mitotic progression. Screening a HeLa cell cDNA expression library with this antibody has been shown to yield a cDNA predicted to encode a protein significantly related within its amino terminal half to the motor ends of members of the kinesin superfamily. Since this similarity does not extend further, it has been suggested that the CHO1 antigen, now designated MKLP-1 (mitotic kinesin-like protein-1), represents a novel kinesin. Sequence analysis has also been shown to predict that MKLP-1 possesses features typical of nuclear proteins. Immunocytological studies have demonstrated that MKLP-1 moves from the nucleus early in mitosis and then to the midbody after cytokinesis. MKLP-1 has been shown to bundle antiparallel microtubules in vitro and to move them at rates comparable to spindle elongation in vivo. A hamster homolog of MKLP-1, designated CHO1 antigen, has also been isolated. Although apparently functionally equivalent with respect to microtubule bundling activity, there are significant differences between the human and hamster proteins at their C-termini, possibly due to alternative splicing or the presence of more than one MKLP-1 locus.