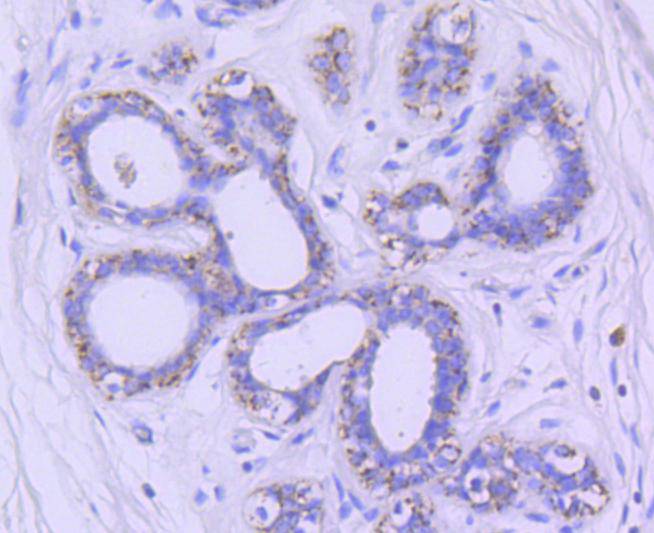
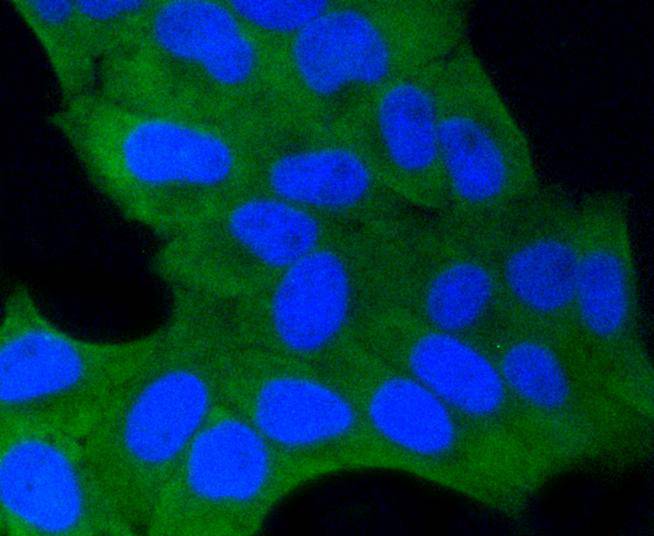
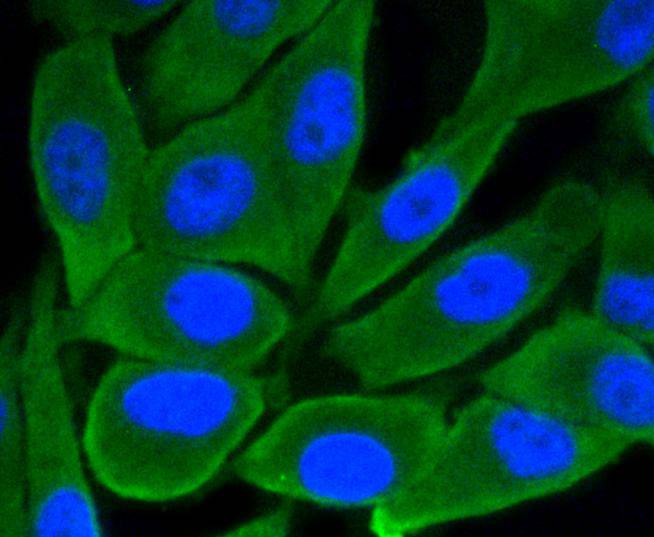
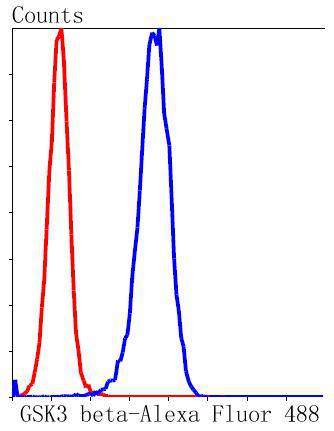
Glycogen synthase kinase 3, or GSK-3, is a serine/threonine, proline-directed kinase involved in a diverse array of signaling pathways, including glycogen synthesis and cellular adhesion, and has been implicated in Alzheimers disease. Two forms of GSK-3, designated GSK-3α and GSK-3β, have been identified and differ in their subcellular localization. Tau, a microtubule-binding protein which serves to stabilize microtubules in growing axons, is found to be hyper-phosphorylated in paired helical filaments (PHF), the major fibrous component of neurofibrillary lesions associated with Alzheimers disease. Hyperphosphorylation of Tau is thought to be the critical event leading to the assembly of PHF. Six Tau protein isoforms have been identified, all of which are phosphorylated by GSK-3. This presents the possibility that miscues in GSK-3 signaling contribute to the onset of Alzheimers disease.