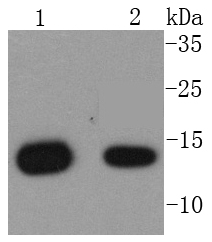
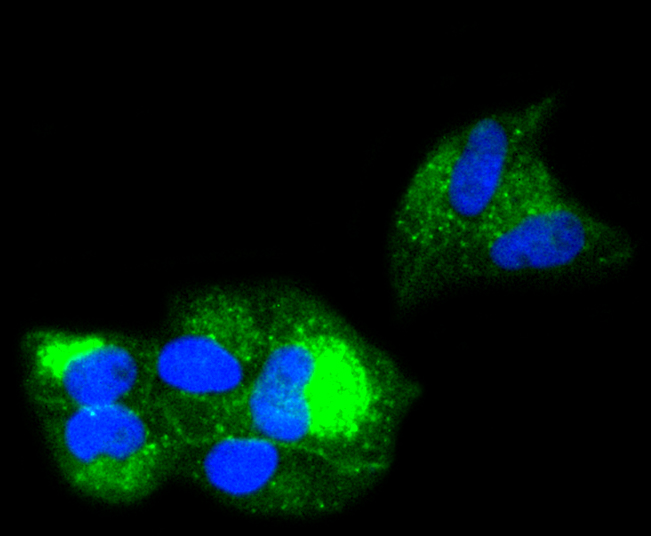
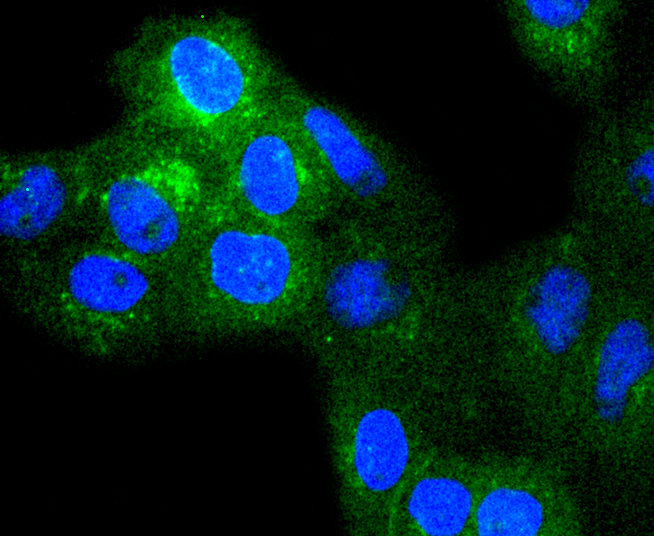
Major histocompatibility complex (MHC) class 1 molecules bind to antigens for presentation on the surface of cells. The proteasome is responsible for producing these antigens from the components of foreign pathogens. MHC class 1 molecules consist of an a heavy chain that contains three subdomains (α1, α2, α3), and a non-covalent associating light chain, known as β-2-Microglobulin. β-2-Microglobulin associates with the a3 subdomain of the a heavy chain and forms an immunoglobulin domain-like structure that mediates proper folding and expression of MHC class 1 molecules. The α1 and α2 domains of the a heavy chain form the peptide antigen-binding cleft. Mice that lack β-2-Microglobulin protein show a normal distribution of T cells, yet have no mature CD4-8+ T cells and are defective in CD4-8+ T cell-mediated cytotoxicity. Interferon-γ can stimulate production of β-2-Microglobulin transcripts. The human β-2-Microglobulin gene maps to chromosome 15q21-q22.2 and encodes a 119 amino acid protein. Mutations in the β-2-Microglobulin gene can enhance the progression of malignant melanoma phenotypes.