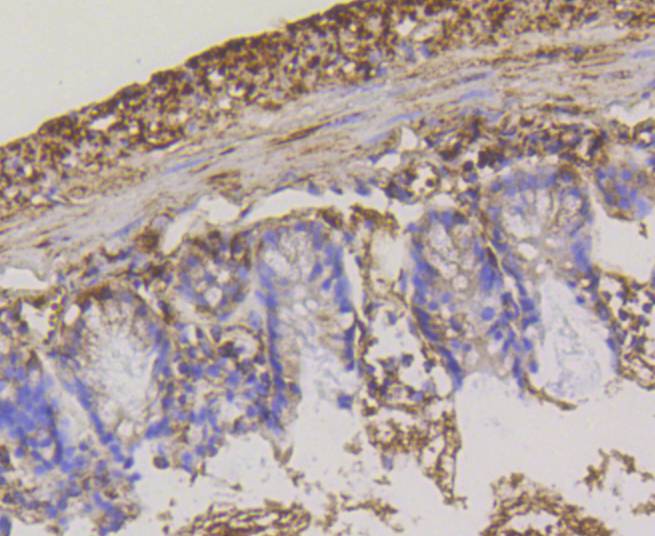
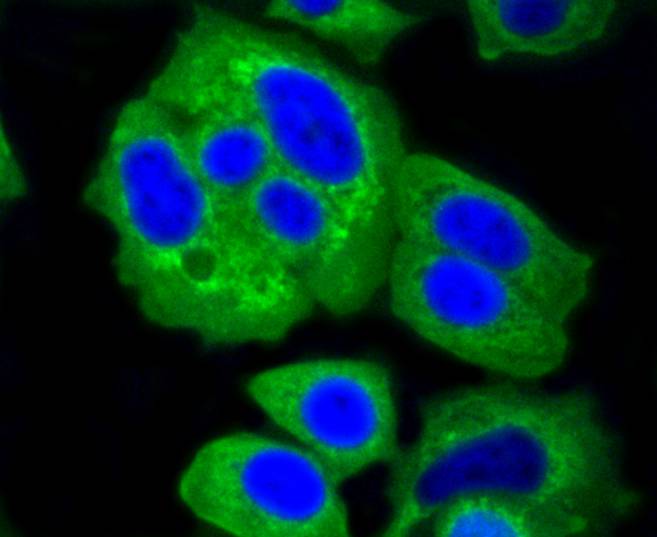
The hexokinases utilize Mg-ATP as a phosphoryl donor to catalyze the first step of intracellular glucose metabolism, the conversion of glucose to glucose-6-phosphate. Four hexokinase isoenzymes have been identified, including hexokinase I (HXK I), hexokinase II (HXK II), hexokinase III (HXK III) and hexokinase IV (HXK IV, also designated glucokinase or GCK). Hexokinases I-III each contain an N-terminal cluster of hydrophobic amino acids. Glucokinase lacks the N-terminal hydrophobic cluster. The hydrophobic cluster is thought to be necessary for membrane binding. This is substantiated by the finding that glucokinase has lower affinity for glucose than do the other hexokinases. HXK I has been shown to be expressed in brain, kidney and heart tissues as well as in hepatoma cell lines. HXK II is involved in the uptake and utilization of glucose by adipose and skeletal tissues. Of the hexokinases, HXK III has the highest affinity for glucose. Glucokinase is expressed in pancreatic beta cells where it functions as a glucose sensor, determining the set point for insulin secretion.