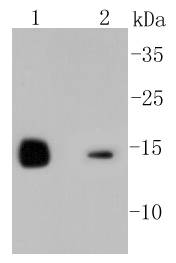
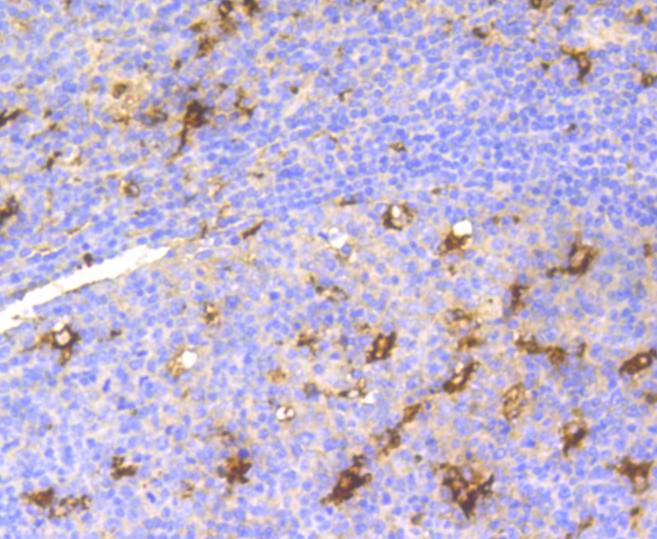
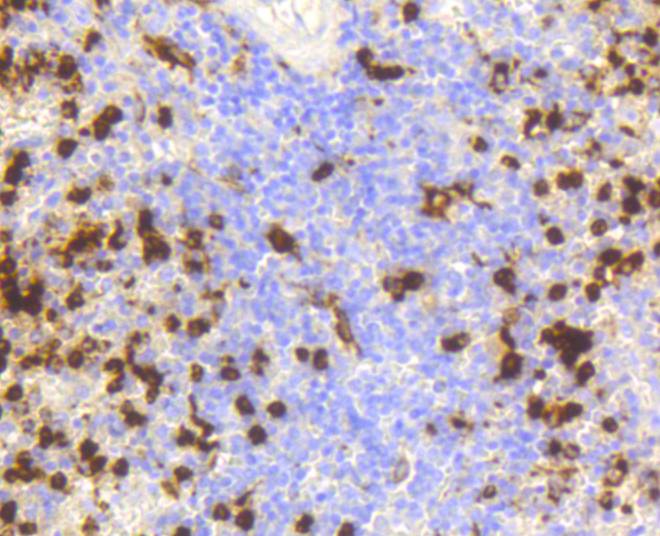
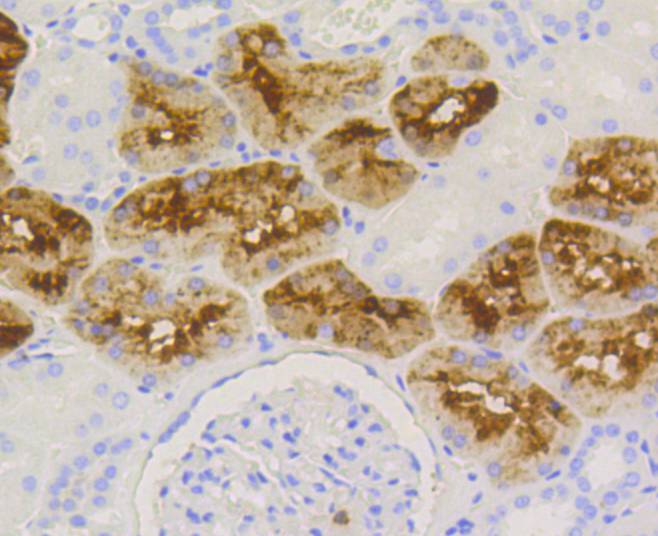
The origins of the lysozyme proteins date back an estimated 400 to 600 million years. Generally, lysozyme genes are relatively small, roughly 10 kilobases in length, and composed of four exons and three introns. Originally a bacteriolytic defensive agent, the function of this family of proteins adapted to serve a digestive function in its present forms. Lysozymes in tissues and body fluids are associated with the monocyte-macrophage system and enhance the activity of immunoagents. Lysozyme C belongs to the glycosyl hydrolase 22 family, and newly identified relatives of Lysozyme C appear to possess anti-HIV activity, as well as preserved bacteriolytic function against Micrococcus lysodeikticus. Lysozyme C is capable of both hydrolysis and transglycosylation and also a slight esterase activity. It acts rapidly on both peptide-substituted and unsubstituted peptidoglycan, and slowly on chitin oligosaccharides. Lysozyme C defects are a cause of amyloidosis VIII, also called familial visceral or Ostertag-type amyloidosis.