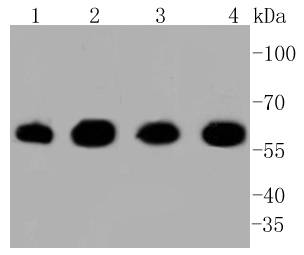
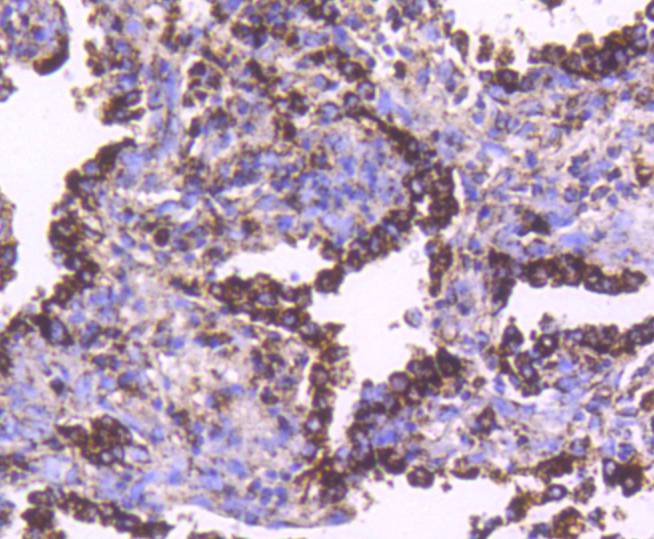
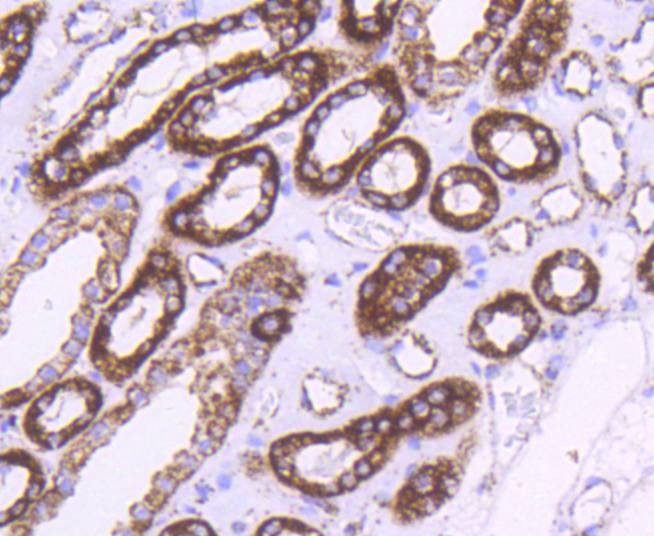
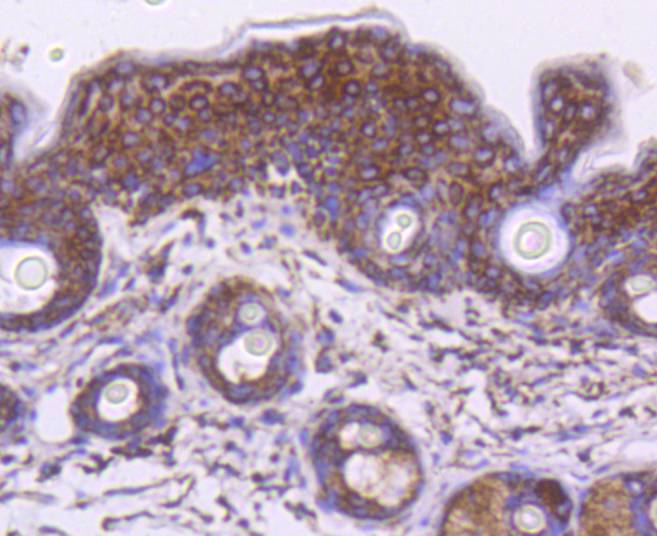
The heat shock proteins (HSPs) comprise a group of highly conserved, abundantly expressed proteins with diverse functions, including the assembly and sequestering of multiprotein complexes, transportation of nascent poly-peptide chains across cellular membranes, and the regulation of protein folding. HSPs (also known as molecular chaperones) fall into six general families: HSP 90, HSP 70, HSP 60, the low molecular weight HSPs, the immunophilins, and the HSP 110 family. The constitutively expressed mitochondrial protein HSP 60 shares the ability to recognize and stabilize proteins during folding, assembly and disassembly with other HSP family members. The mitochondrial and cytosolic localization of HSP 60, combined with its binding and catalysis of folding of newly synthesized proteins destined for the mitochondrial matrix, classify this protein as a molecular chaperone. An additional role of HSP 60 is to act as a cell surface marker for T cell recognition.