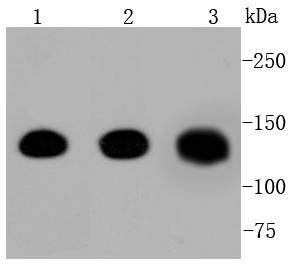
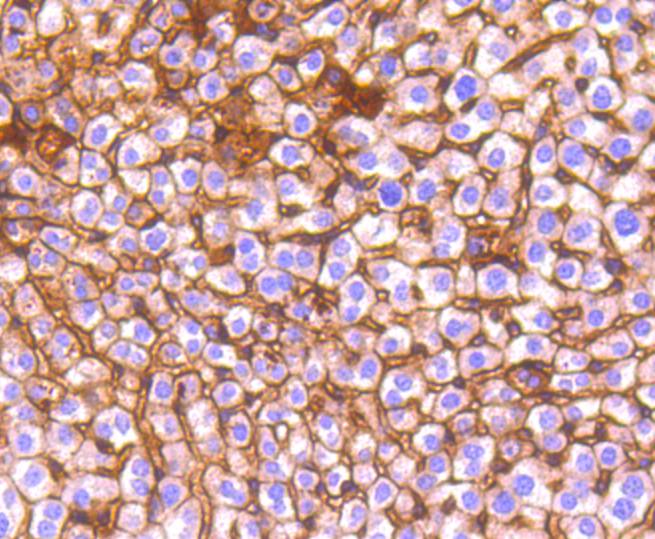
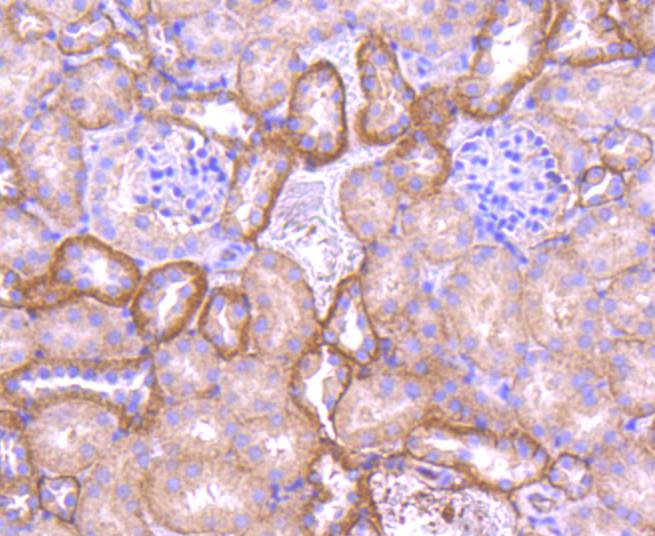
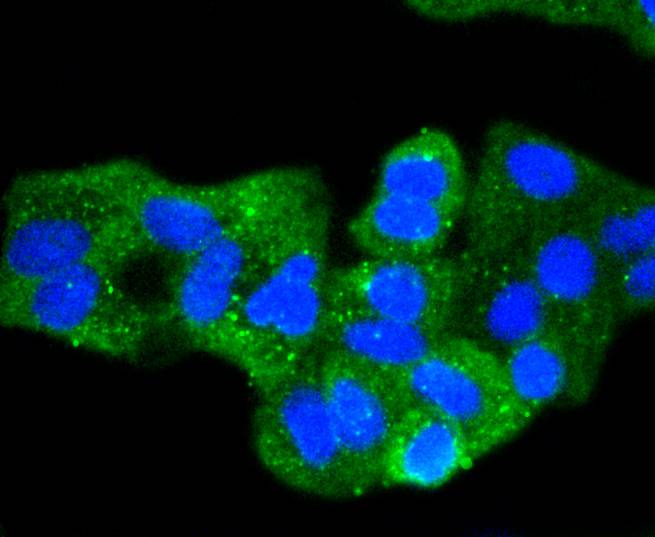
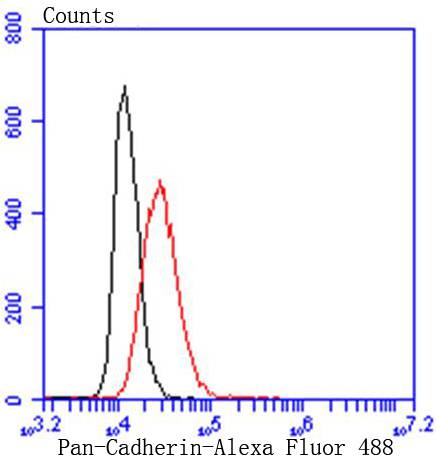
Cadherins comprise a family of Ca2+-dependent adhesion molecules that function to mediate cell-cell binding critical to the maintenance of tissue structure and morphogenesis. The classical cadherins, E-, N- and P-cadherin, consist of large extracellular domains characterized by a series of five homologous NH2 terminal repeats. The most distal of these cadherins is thought to be responsible for binding specificity, transmembrane domains and carboxy terminal intracellular domains. The relatively short intracellular domains interact with a variety of cytoplasmic proteins, such as -catenin, to regulate cadherin function. Members of this family of adhesion proteins include rat cadherin K (and its human homolog, cadherin, R-cadherin, B-cadherin, E/P cadherin and cadherin-5.