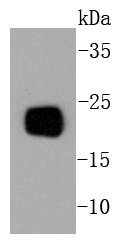
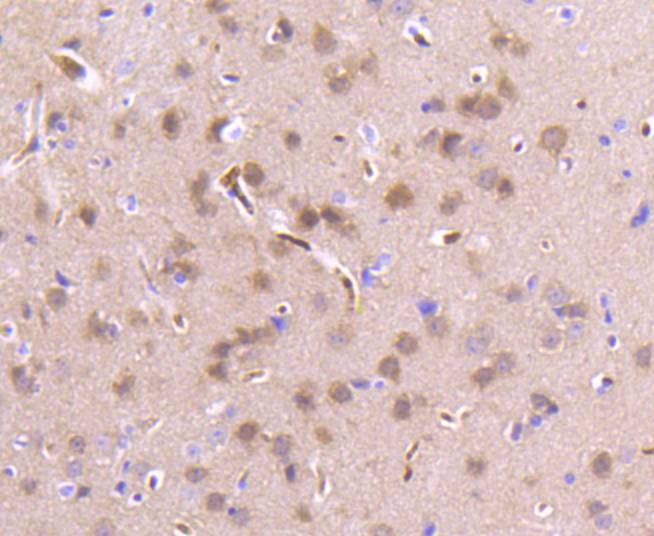
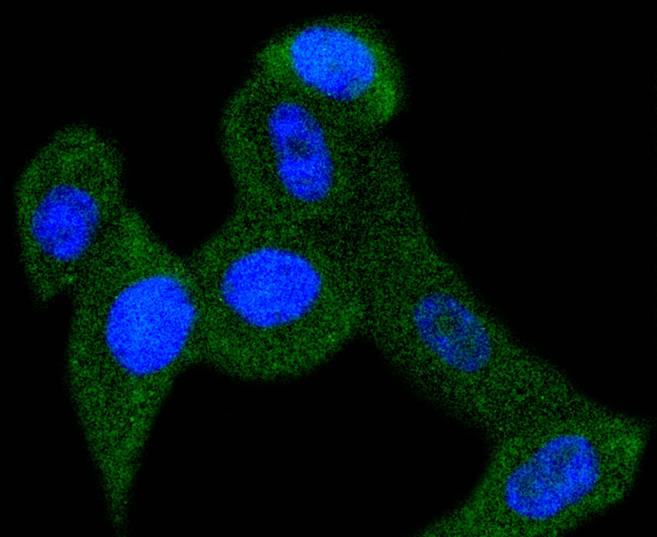
Members of the a-chemokine subfamily of inducible, secreted, pro-inflammatory cytokines contain a similar motif, in which the first two cysteine residues are separated by a single residue (Cys-X-Cys), and are also chemotactic for neutrophils. The platelet basic protein (PBP), a member of the a(lpha)-chemokine family, resides in the a(lpha)-granules of platelets and is released upon their activation. Proteolytic cleavage of the amino terminus of PBP leads to the generation of several peptides, which include mature PBP, connective tissue-activating peptide III (CTAP III, also designated low affinity platelet factor IV (LA-PF4)), b-thromboglobulin (b-TG), and neutrophil-activating peptide 2 (NAP-2). PBP and its N-truncated derivatives mediate inflammation and wound healing. Specifically, NAP-2 activates chemotaxis and degranulation in neutrophils during inflammation. The gene encoding human PBP maps to chromosome 4q12-q13.