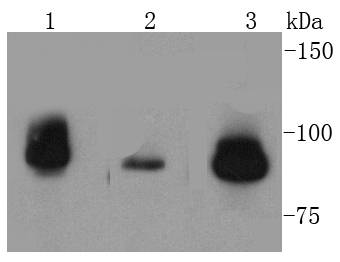
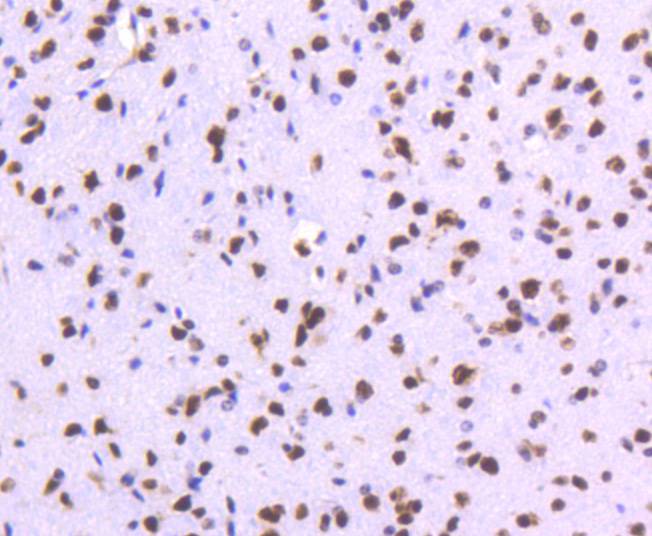
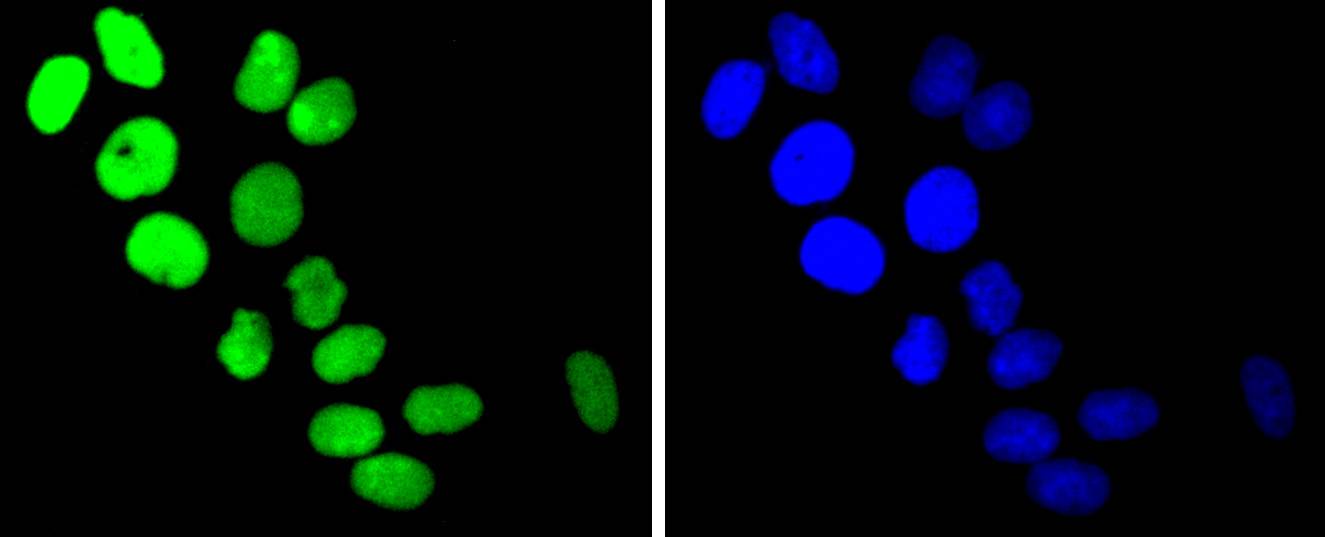
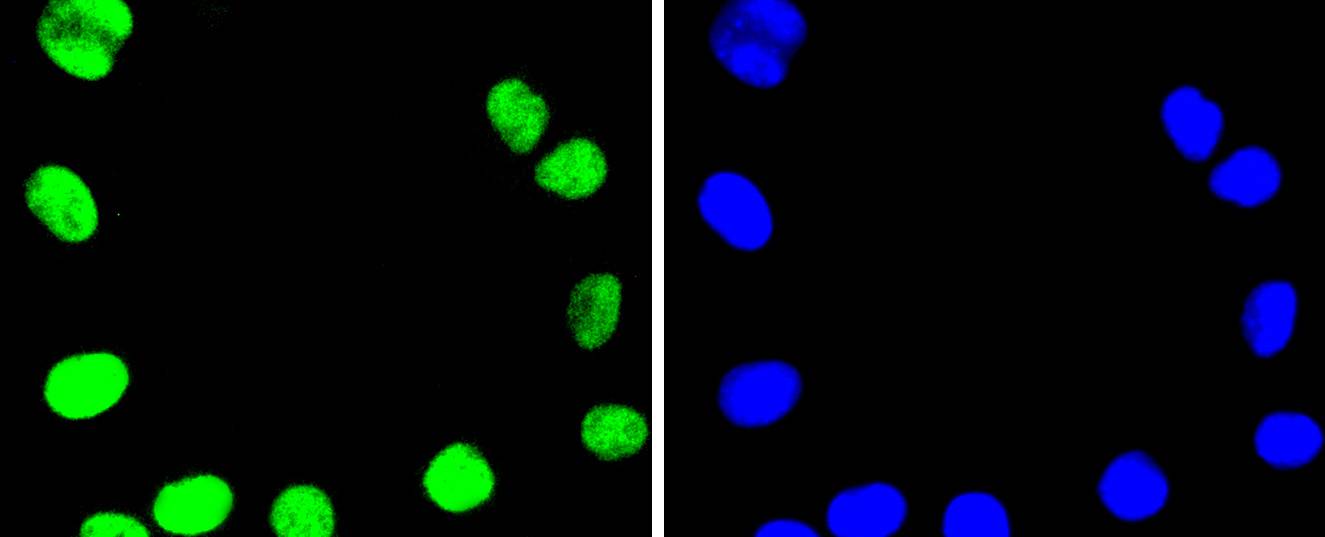
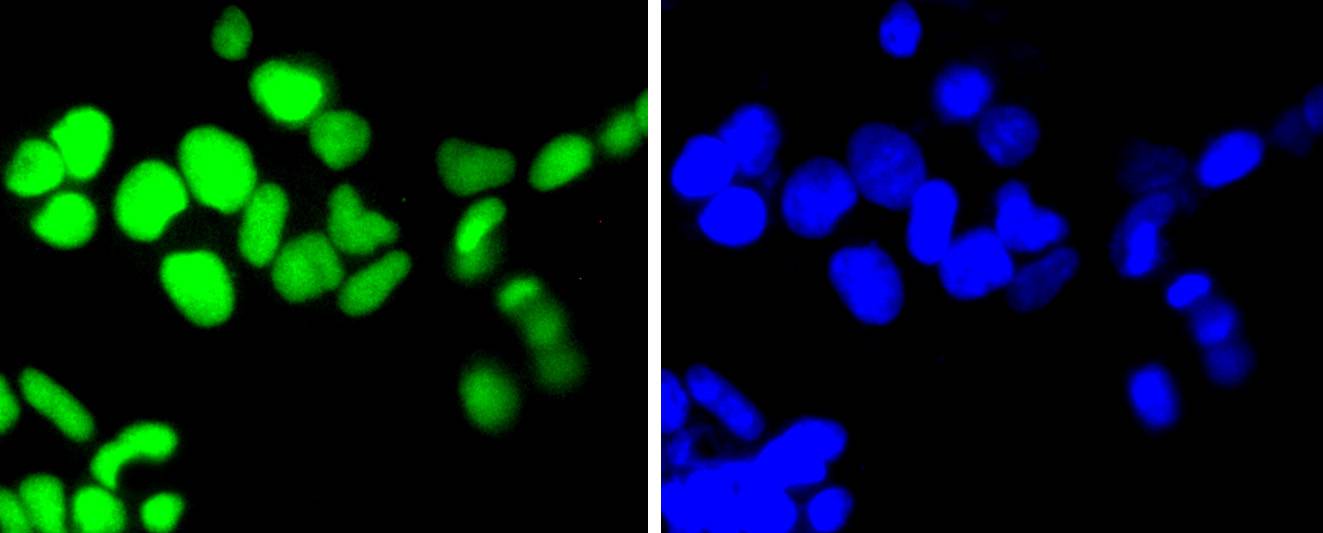
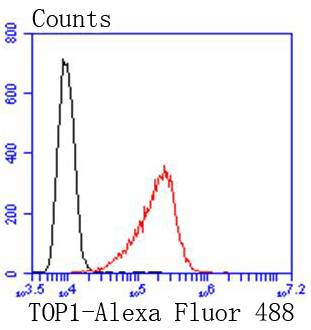
DNA topoisomerases play essential roles in many DNA metabolic processes including DNA repair. Topoisomerases can introduce DNA damage upon exposure to drugs that stabilize the covalent protein-DNA intermediate of the topoisomerase reaction. Lesions in DNA are also able to trap topoisomerase-DNA intermediates. DNA topoisomerase I (Top1) catalyzes the relaxation of supercoiled DNA by a mechanism of transient DNA strand cleavage characterized by the formation of a phosphotyrosyl bond between the DNA end and active site tyrosine. The antitumor agent camptothecin (CPT) reversibly stabilizes the covalent enzyme-DNA intermediate by inhibiting DNA religation. When a replication fork collides with a DNA Top1 cleavage complex, the covalently bound enzyme must be removed from the DNA 3' end before recombination-dependent replication restart. The tyrosyl-DNA phosphodiesterase Tdp1 and the structure-specific endonuclease Rad1-Rad10 function as primary alternative pathways of Top1 repair in Saccharomyces cerevisiae. In the budding yeast S. cerevisiae, DNA topoisomerases I and II can functionally substitute for each other in removing positive and negative DNA supercoils.