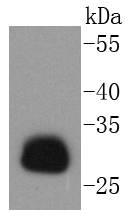
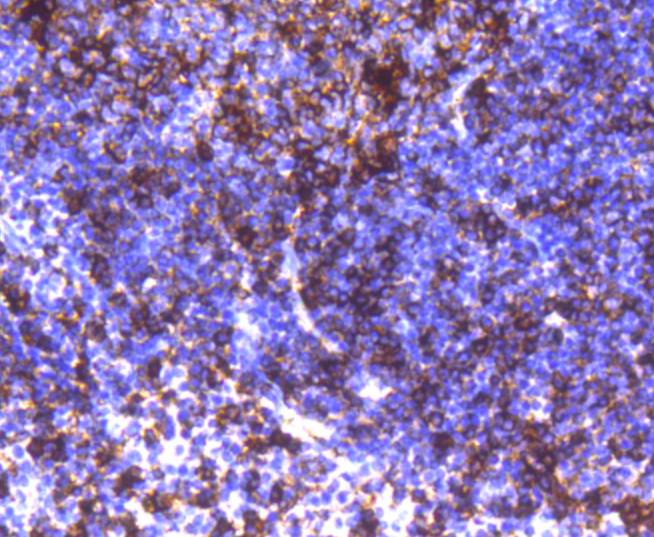
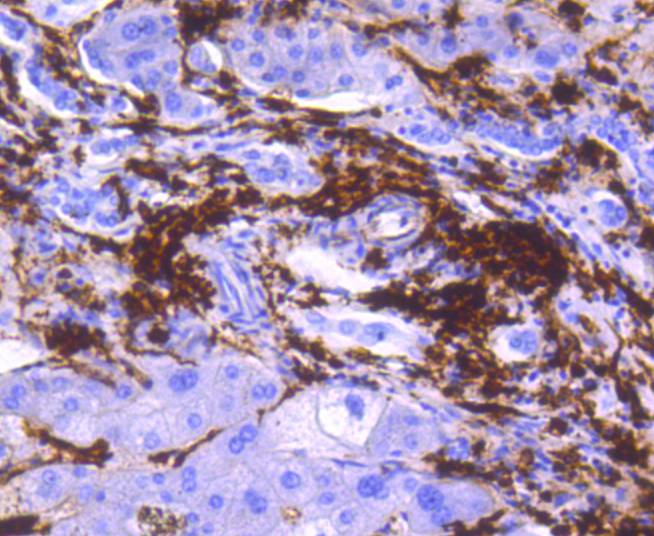
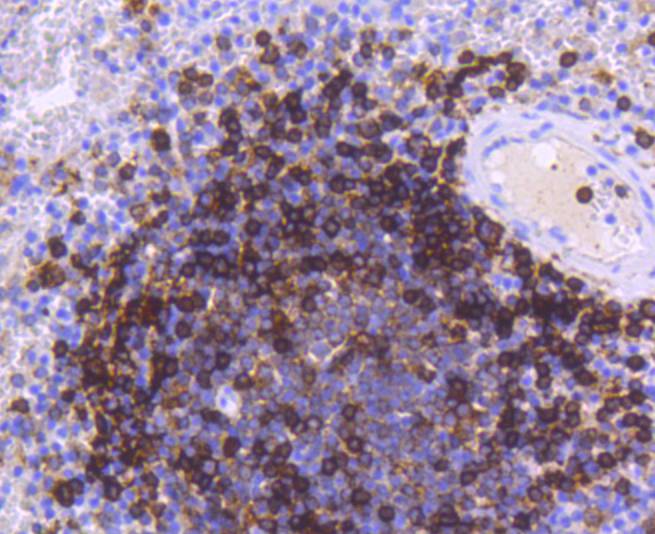
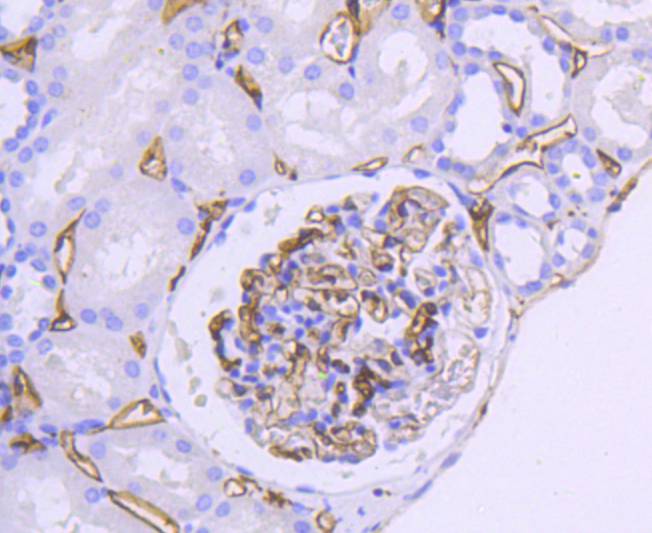
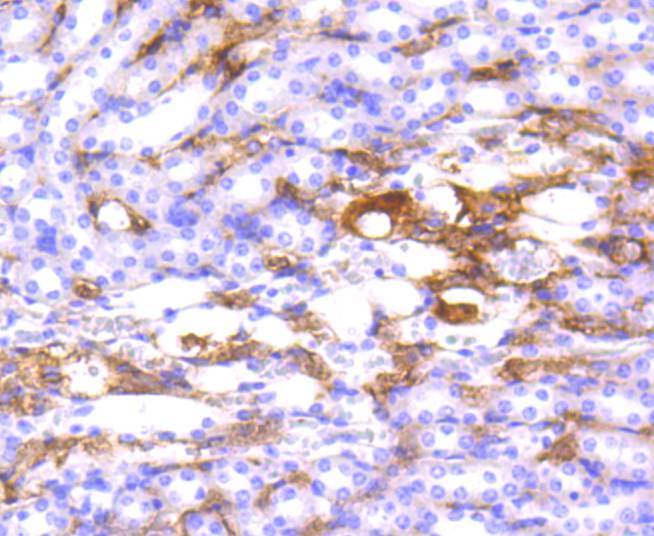
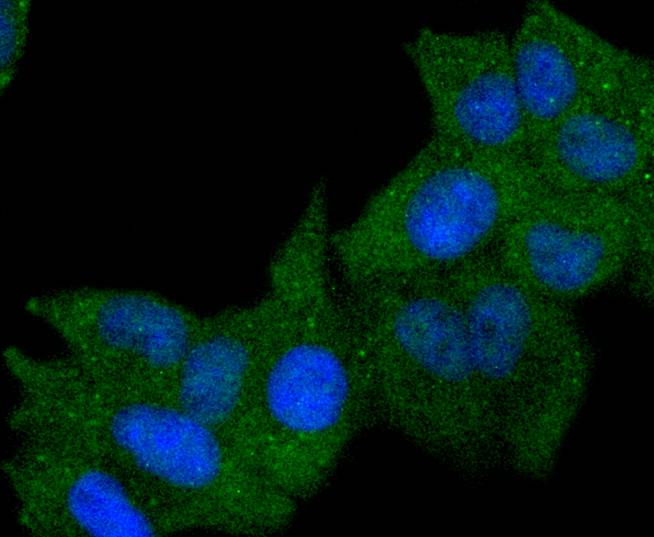
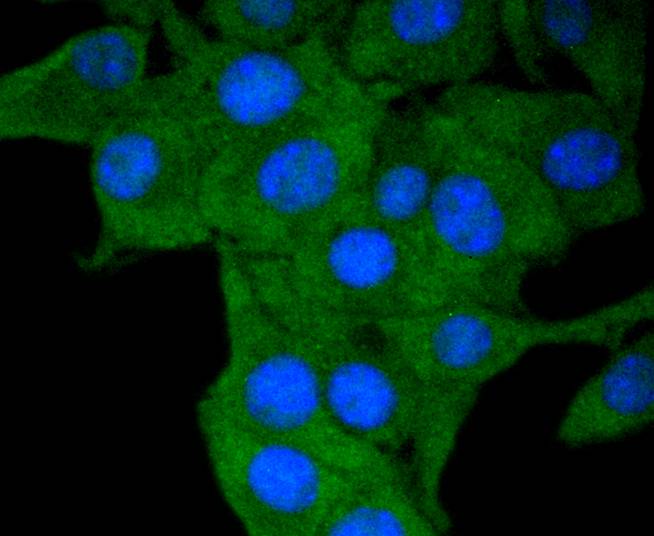
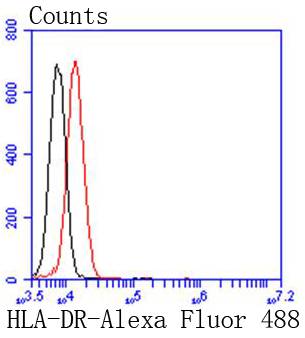
Major histocompatibility complex (MHC) class II molecules destined for presentation to CD4+ helper T cells is determined by two key events. These events include the dissociation of class II-associated invariant chain peptides (CLIP) from an antigen binding groove in MHC II α/β dimers through the activity of MHC molecules HLA-DM and -DO, and subsequent peptide antigen binding. Accumulating in endosomal/lysosomal compartments and on the surface of B cells, HLA-DM, -DO molecules regulate the dissociation of CLIP and the subsequent binding of exogenous peptides to HLA class II molecules (HLA-DR, -DQ and -DP) by sustaining a conformation that favors peptide exchange. RFLP analysis of HLA-DM genes from rheumatoid arthritis (RA) patients suggests that certain polymorphisms are genetic factors for RA susceptibility. HLA-B belongs to the HLA class I heavy chain paralogs. Class I molecules play a central role in the immune system by presenting peptides derived from the endoplasmic reticulum lumen. HLA-B and -C can form heterodimers consisting of a membrane anchored heavy chain and a light chain (β-2-Microglobulin). Polymorphisms yield hundreds of HLA-B and -C alleles.