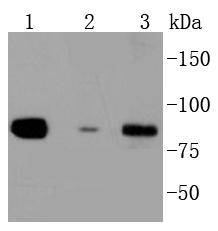
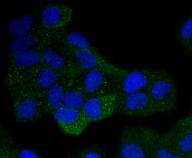
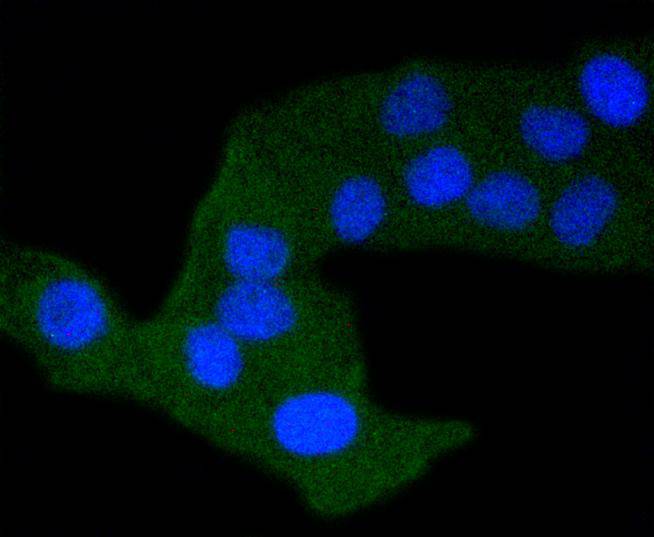
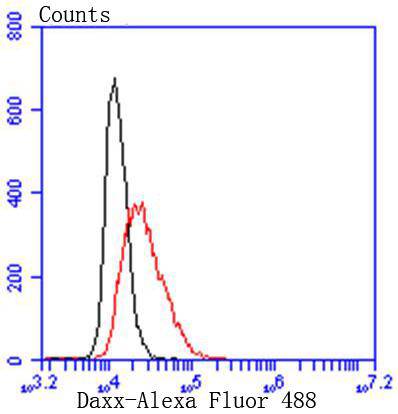
Activation of the cell surface receptor FAS by FAS ligand leads to the initiation of apoptosis, a process necessary for the regulation of the immune system and tissue homeostasis. FAS-mediated apoptosis appears to involve a number of divergent and overlapping pathways. Daxx appears to be a central component of a FAS-mediated apoptotic pathway involving the activation of Jun N-terminal kinase (JNK). Although Daxx itself does not contain a death domain, it specifically binds to the death domain of FAS. Overexpression of Daxx activates the JNK pathway and enhances FAS-mediated apoptosis. The Daxx apoptotic pathway acts cooperatively with but is distinct from the FAS-mediated pathway that involves interactions between the death domain-containing protein FADD and the cysteine protease FLICE. Unlike the FAS-FADD-FLICE pathway, the Daxx pathway is sensitive to the apoptotic inhibitor protein Bcl-2.