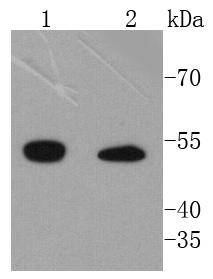
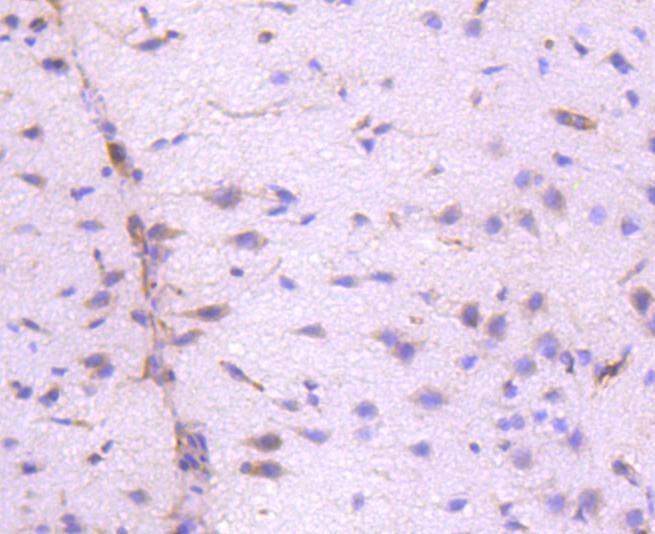
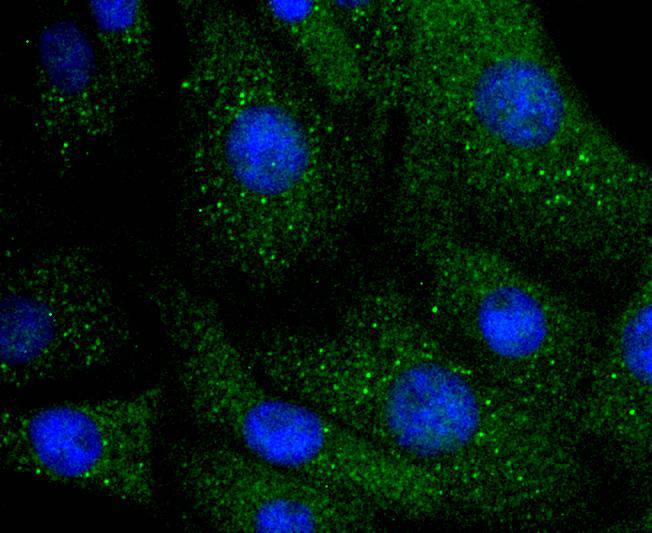
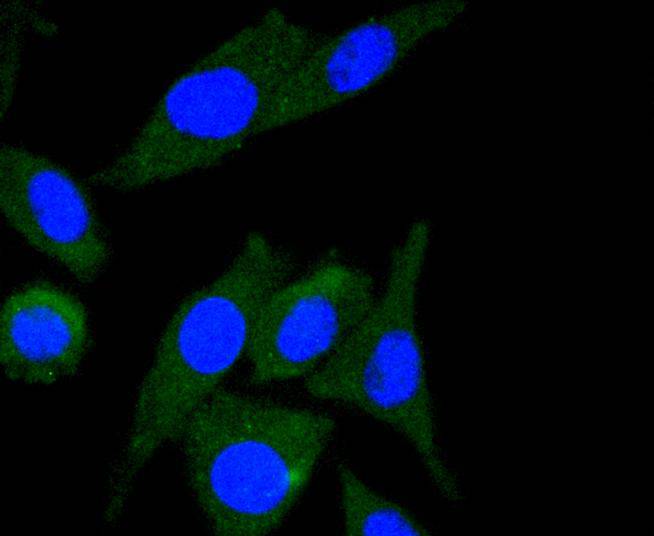
Integrins are heterodimers composed of non-covalently associated transmembrane a and b subunits. The 16 a and 8 b subunits heterodimerize to produce more than 20 different receptors. Most integrin receptors bind to ligands that are components of the extracellular matrix. Certain integrins can also bind to soluble ligands such as Fibrinogen, or to counterreceptors on adjacent cells, such as the intracellular adhesion molecules (ICAMs), leading to aggregation of cells. In addition to mediating cell adhesion and cytoskeletal organization, integrins function as signaling receptors. Signals transduced by integrins play a role in many biological processes, including cell growth, differentiation, migration and apoptosis. ILK (integrin-linked kinase) was identified as a serine/threonine kinase that phosphorylates b1 and b3 integrins. ILK expression has been shown to be reduced in response to Fibronectin, a known integrin ligand. Overexpression of ILK was shown to upregulate the Fibronectin matrix assembly in epithelial cells, indicating a potential role for ILK in cell growth, cell survival and tumorigenesis.