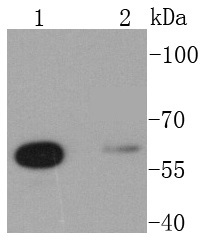
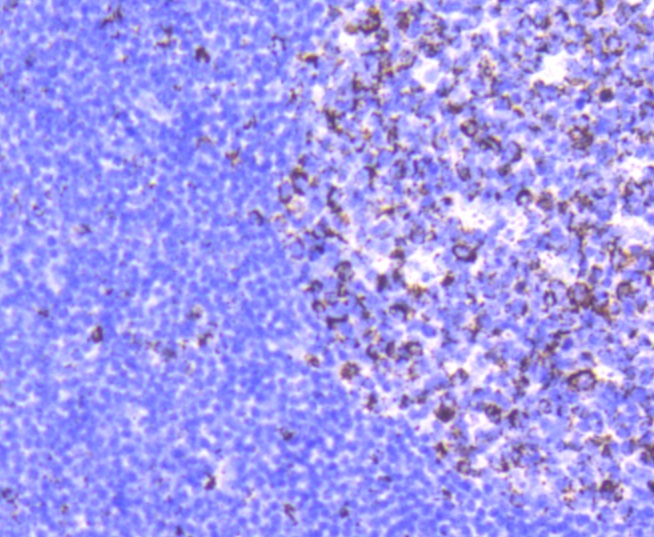
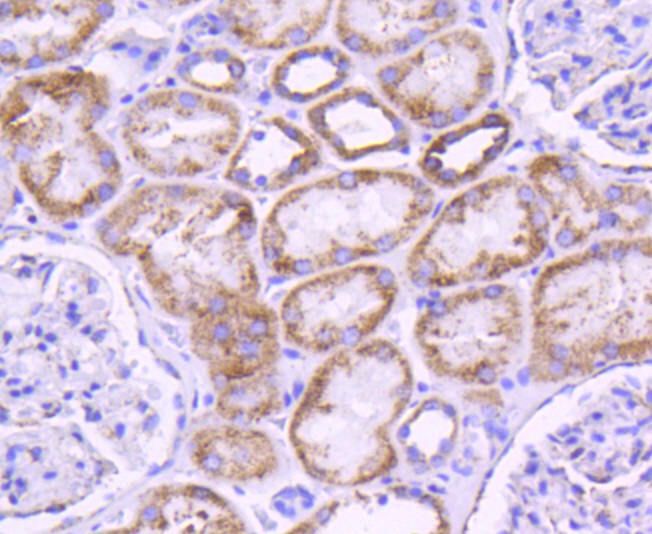
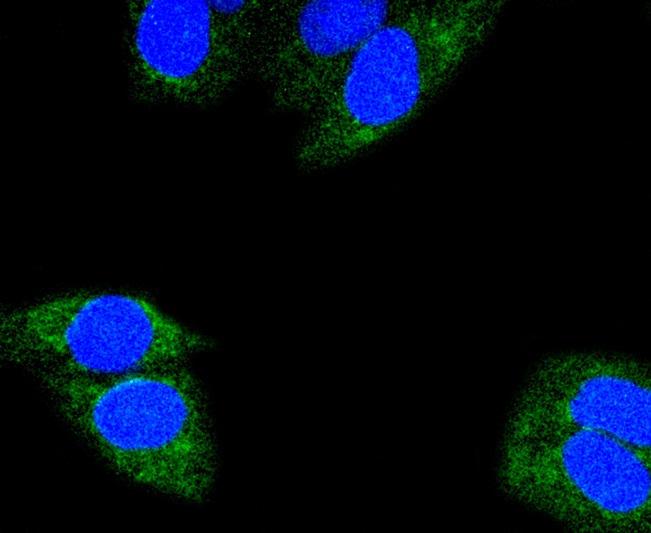
Glutamine is an important molecule involved in several cellular functions, including nitrogen and carbon transport, hepatic urea synthesis, renal ammoniagenesis, and gluconeogenesis. Glutamine is catabolized by either the liver-type (LGA) or kidney-type (KGA) glutaminase. KGA is mitochondrial specific protein whose expression in kidney is increased during metabolic acidosis. This process is mediated by an 8-base AU-sequence in KGA that functions as a pH-response element. The human KGA gene maps to chromosome 2, and produces three isoforms, designated KGA, GAC, and GAM, by alternative splicing. KGA is synthesized as a cytosolic protein that is transported to the mitochondria as an intermediate protein, and is further cleaved into the KGA isoform and the GAC isoform. The processing of the GAM isoform is unclear. The KGA isoform is abundant in brain and kidney, while the GAC isoform is principally expressed in cardiac muscle and pancreas. The GAM isoform is solely expressed in cardiac and skeletal muscle.