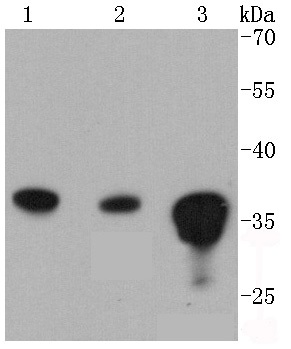
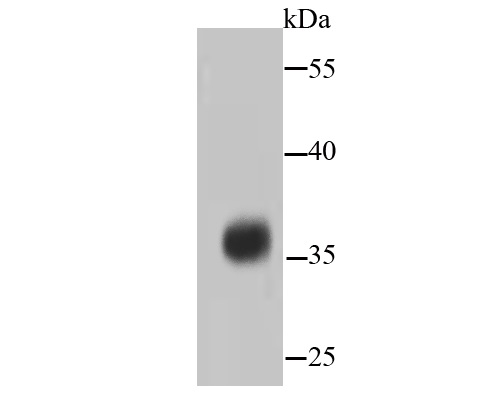
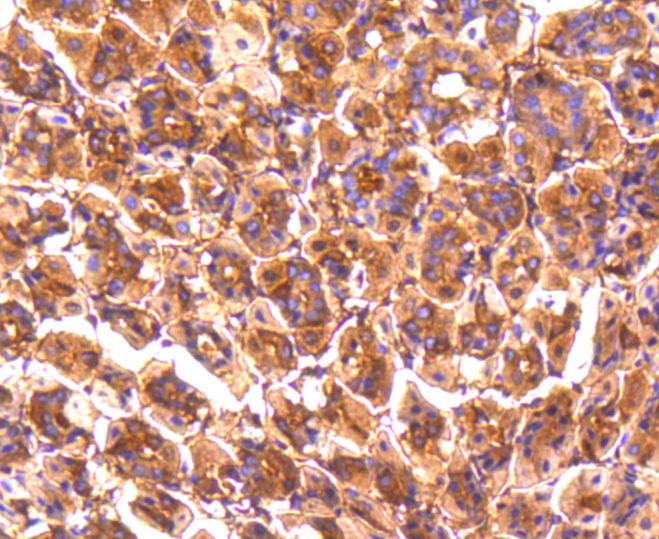
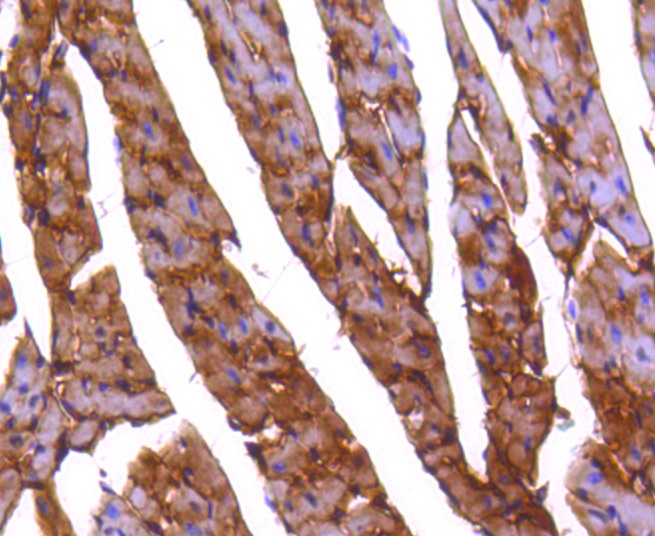
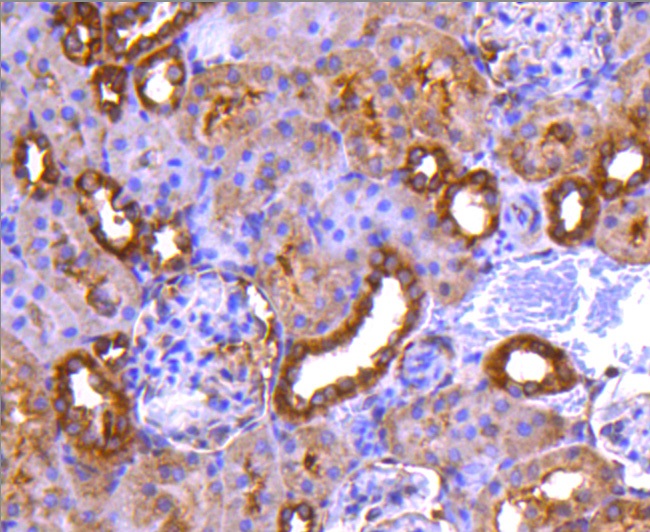
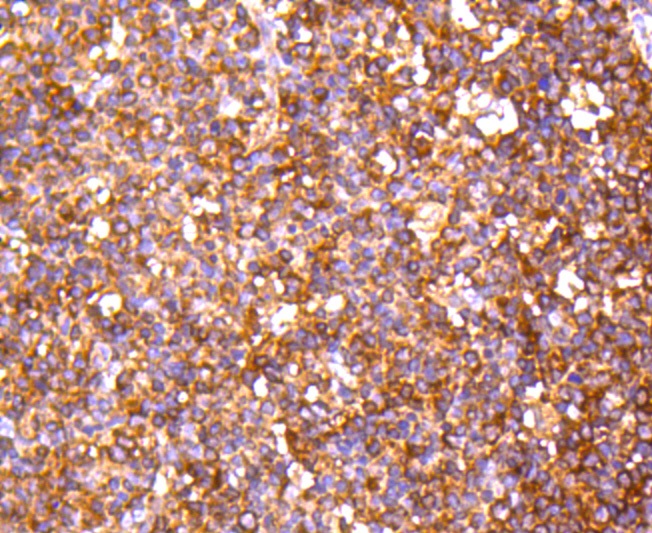
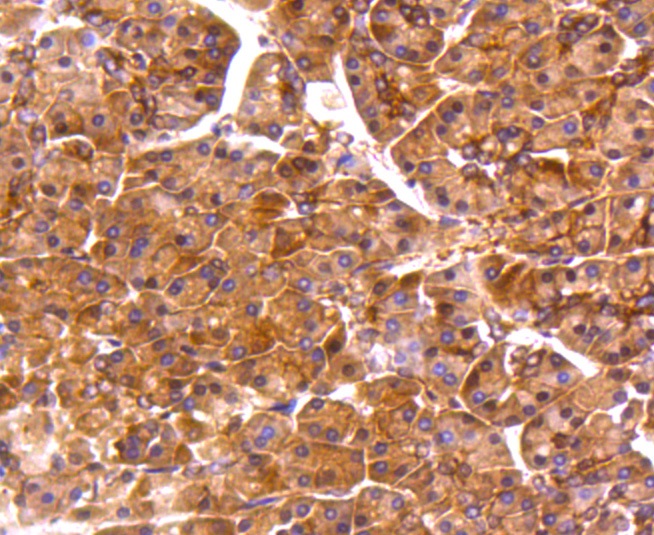
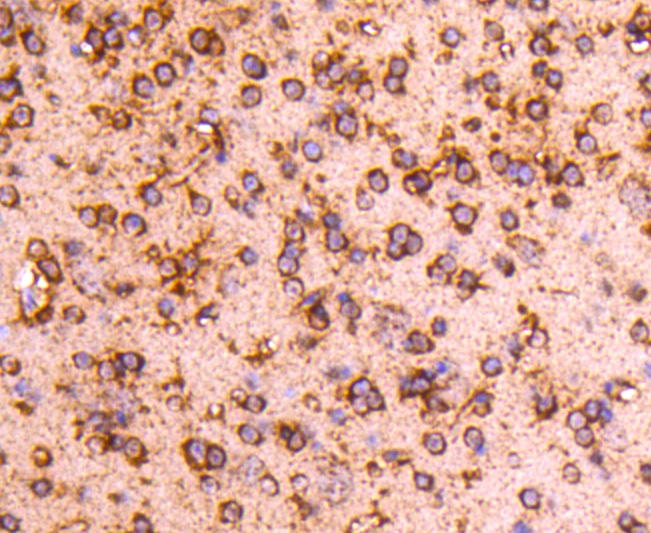
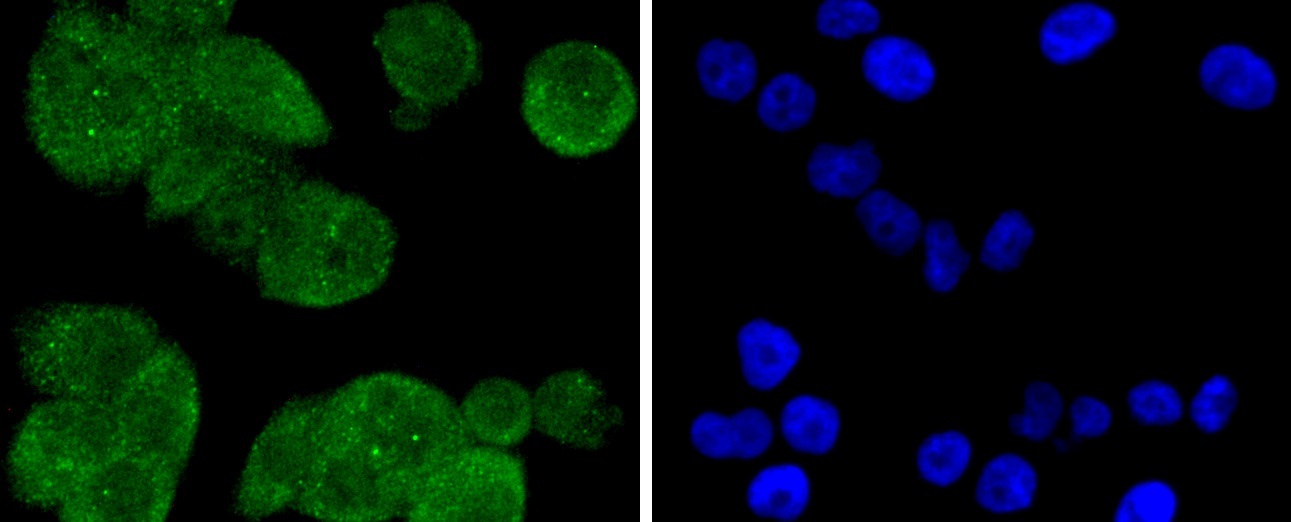
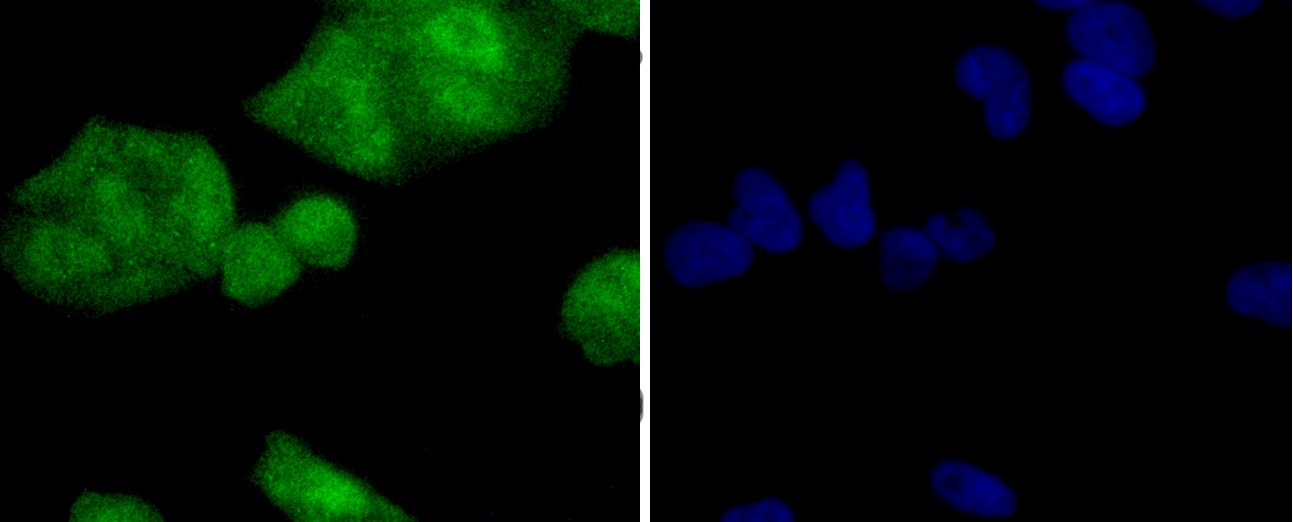
The catalytic subunit of protein phosphatase 2A (PP2A) is inactivated by in vitro phosphorylation of Tyr-307 by receptor and nonreceptor protein tyrosine kinases. The catalytic subunit of PP2A is phosphorylated by tyrosine-specific protein kinases and associates with a variety of regulatory subunits. Phosphorylation is enhanced in the presence of the phosphatase inhibitor okadaic acid, consistent with an autodephosphorylation reaction. Phosphorylation is catalyzed by p60v-src, p56lck, epidermal growth factor receptors, and insulin receptors. Transient deactivation of PP2A might enhance transmission of cellular signals through kinase cascades within cells. In eukaryotes, the phosphorylation and dephosphorylation of proteins on serine and threonine residues is an essential means of regulating a broad range of cellular functions, including cell division, homeostasis and apoptosis. A group of proteins that are intimately involved in this process are the protein phosphatases.