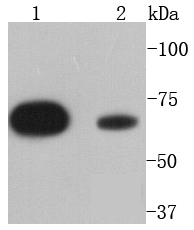
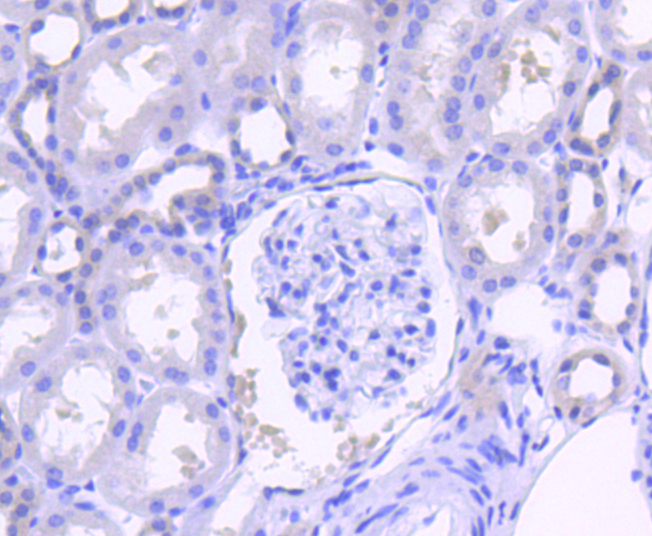
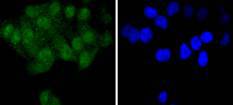
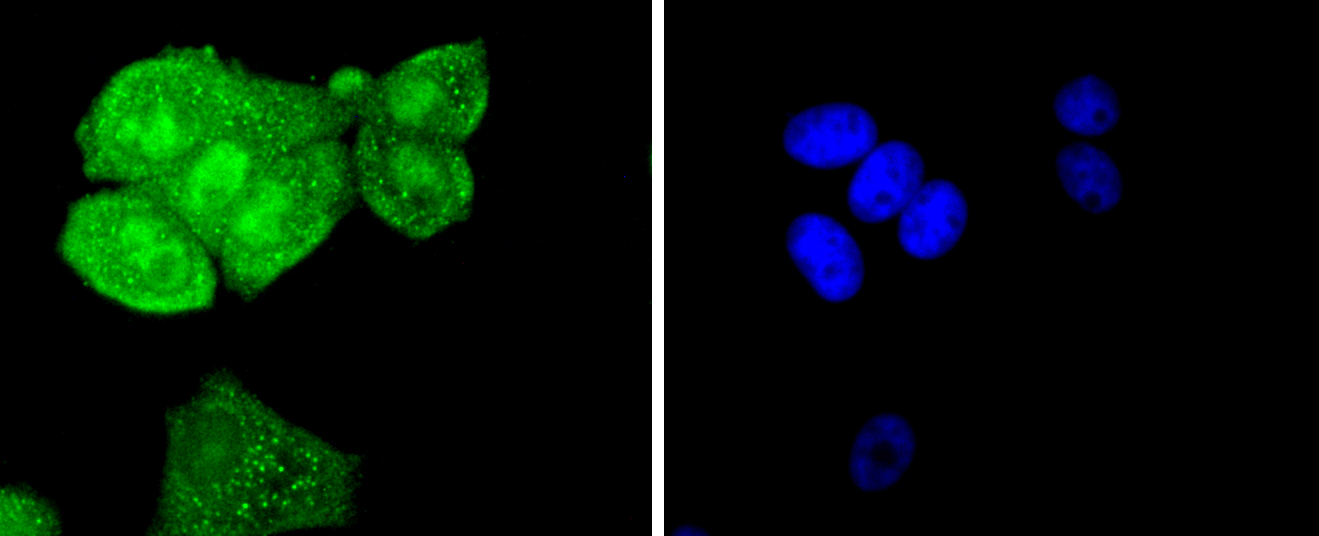
The steady state of protein tyrosyl phosphorylation in cells is regulated by the opposing action of tyrosine kinases and protein tyrosine phosphatases (PTPs). Several groups have independently identified a non-transmembrane PTP, designated SH-PTP1 (also known as PTP1C, HCP and SHP), which is primarily expressed in hematopoietic cells and characterized by the presence of two SH2 domains N-terminal to the PTP domain. SH2 domains generally mediate the association of regulatory molecules with specific phosphotyrosine-containing sites on autophosphorylated receptors, thereby controlling the initial interaction of receptors with these substrates. A second and much more widely expressed PTP with SH2 domains, SH-PTP2 (also designated PTP1D and Syp), has been identified. Strong sequence similarity between SH-PTP2 and the Drosophila gene corkscrew (CSW) and their similar patterns of expression suggest that SH-PTP2 is the human corkscrew homolog.