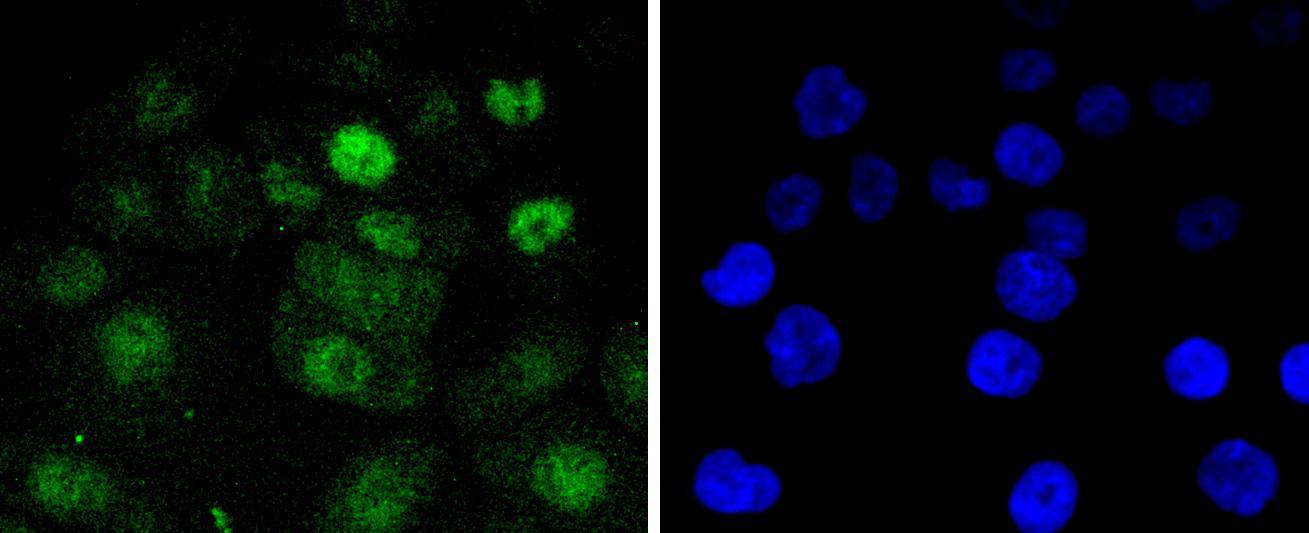
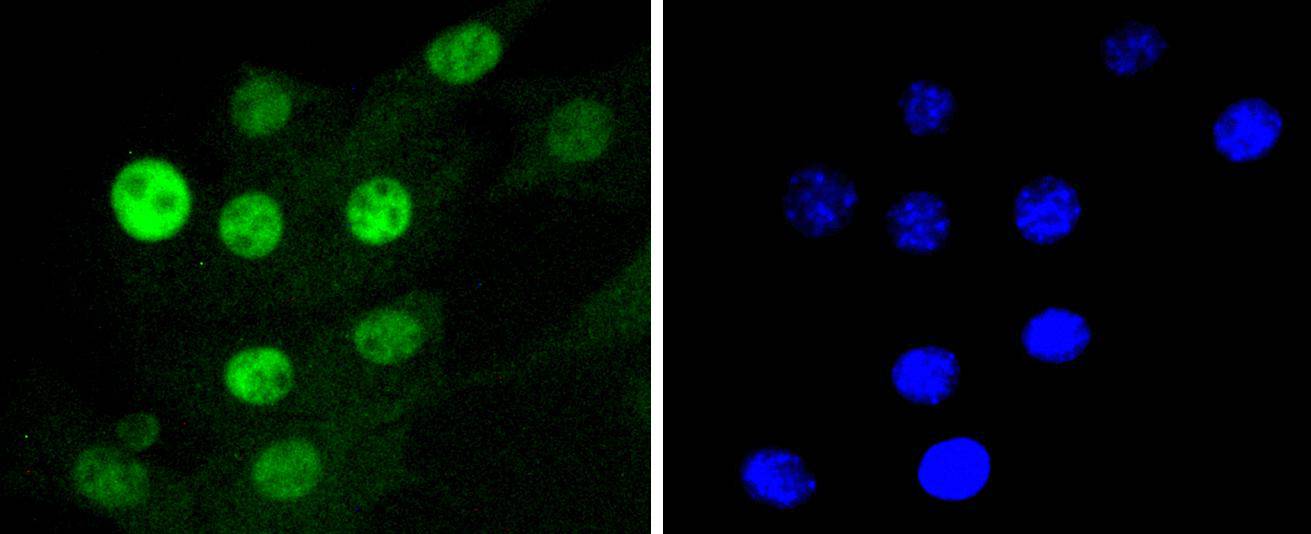
Cell cycle progression is controlled in part by a family of cyclin proteins and cyclin dependent kinases (Cdks). Cdk proteins work in concert with the cyclins to phosphorylate key substrates involved in each phase of cell cycle progression. Another family of proteins, Cdk inhibitors, also plays a role in regulating the cell cycle by binding to cyclin-Cdk complexes and modulating their activity. Several Cdk proteins have been identified, including Cdk2-Cdk8, PCTAIRE-1-PCTAIRE-3, PITALRE and PITSLRE. Cdk4, in complex with D-type cyclins, is thought to regulate cell growth during the G1 phase of the cell cycle. This association with a D-type cyclin upregulates Cdk4 activity, whereas binding to the Cdk inhibitor p16 downregulates Cdk4 activity. Activation of the Cdk4-cyclin complexes requires phosphorylation on a single threonyl residue of Cdk4, catalyzed by a Cdk-activating protein (CAK).